 Fig. 1: SEM image of Ni-PTitanium surfaces are always covered by a tenacious titanium oxide film that interferes with the good bonding of plating metal to substrate. The problem is severe when the parts are heat treated. Furthermore, titanium reacts with the oxygen that is produced by many pre-plating processes forming instantaneously a passive oxide film on the underlying substrate. The common alkaline cleaning or acid etching methods are not sufficient to remove it. Plating on titanium has therefore long been considered extremely difficult. For a successful plating on titanium, the chosen process must ensure to complete removal of this native oxide before proceeding for plating. The conventional method of plating on titanium alloys involves the chemical etching, dichromate activation, copper striking and nickel electroplating. The process does not yield satisfactory uniform deposits on intricate parts, and is in particular not suitable for Ti 6Al-4V alloy, where a dull deposit with inadequate adhesion is formed. The pickling solutions based on nitric and hydrofluoric acids are generally effective to remove the titanium oxide film. After removing the oxide film, a cycle of Woods nickel strike or immersion zincating, and electroless nickel plating can be followed. In this section, processes of electroless nickel, gold plating, and blackening of electroless nickel on titanium alloys are discussed.
Fig. 1: SEM image of Ni-PTitanium surfaces are always covered by a tenacious titanium oxide film that interferes with the good bonding of plating metal to substrate. The problem is severe when the parts are heat treated. Furthermore, titanium reacts with the oxygen that is produced by many pre-plating processes forming instantaneously a passive oxide film on the underlying substrate. The common alkaline cleaning or acid etching methods are not sufficient to remove it. Plating on titanium has therefore long been considered extremely difficult. For a successful plating on titanium, the chosen process must ensure to complete removal of this native oxide before proceeding for plating. The conventional method of plating on titanium alloys involves the chemical etching, dichromate activation, copper striking and nickel electroplating. The process does not yield satisfactory uniform deposits on intricate parts, and is in particular not suitable for Ti 6Al-4V alloy, where a dull deposit with inadequate adhesion is formed. The pickling solutions based on nitric and hydrofluoric acids are generally effective to remove the titanium oxide film. After removing the oxide film, a cycle of Woods nickel strike or immersion zincating, and electroless nickel plating can be followed. In this section, processes of electroless nickel, gold plating, and blackening of electroless nickel on titanium alloys are discussed.
Electroless nickel
Electroless nickel is one of the most important surface modification processes, widely employed in various industrial applications due to its unique physical and chemical properties. Electroless Ni-P alloy coating is most widely used and is usually regarded as synonymous with autocatalytic or 'electroless nickel’. Alloying nickel with phosphorus brings significant improvement in mechanical properties and corrosion resistance. The advantages of the electroless coatings [1–3] include:
- Good throwing power, uniform coating thickness on complex objects, can reach the hidden surfaces of complex parts
- Good adhesion to substrate
- Unlimited thickness conceivable
- Can be applied on both conductive and non-conductive materials
- Low porosity
- High corrosion resistance, not susceptible to stress corrosion cracking in acidic, neutral or alkaline conditions
- Excellent lubricity
- Coatings are harder and the hardness can be further improved by heat treatment
- Good wear and abrasion resistance
- Low coefficient of friction
- Good solderability and brazability characteristics
- Magnetic properties propensity
- Fatigue strength
- Low ductility
- Low labour cost.
There are some disadvantages of electroless system:
- Solutions are more expensive than electrolytic plating
- Baths have limited life due to the formation of reaction by-products
- Deposition rates are slow
- Welding characteristics are poor
- Frequent replenishment and careful analytical control of the bath is required to maintain quality
- Brittleness and reduced integrity, due to formation of intermetallic compounds during heat treatment, their application is restricted in a harsh environment, such as under the conditions of high speed and heavy load.
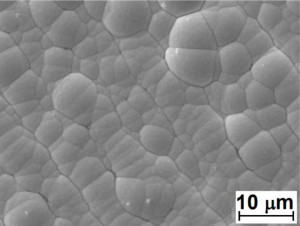
Despite few drawbacks, autocatalytic nickel plating is evolving as an increasingly popular plating choice due to its superior properties, consistency and reliability. The coatings can be further tailor designed with the composition and structure, to offer smart and adaptive solutions for a wide range of applications. Surface morphology of typical Ni-P is shown in Figure 1.
Sharma and Bhojaraj [4] described a process based on immersion zinc/ electroless nickel on titanium alloy Ti6Al4V:
- Solvent degreasing in methyl ethyl ketone for 5–10 minutes.
- Descaling in a solution containing 500 g/L sodium hydroxide and 100 g/L copper sulphate, 1–2 g/L at 90 ºC for 15–20 minutes followed by water rinsing.
- Acid pickling in a solution of 275 ml/L of 70 % nitric acid and 225 ml/L of 40 % hydrofluoric acid for 20–30 seconds.
- Immersion zincating in a solution containing sodium dichromate: 100 g/L, hydrofluoric acid (40 %): 65 ml/L, zinc sulphate: 12 g/L, operating at pH: 2.0 ± 0.2; 90–95 °C, for 3–4 minutes. Water rinsing.
- Stripping the first zincating layer as obtained in the previous step by immersion in acid pickling solution as formulated in step 3 for 45–60 seconds. Water rinse.
- Re-zincating as in step 4 for 5–6 minutes. Water rinse.
- Electroless nickel plating in nickel sulphate: 30 g/L, sodium hypophosphite: 10 g/L, sodium citrate: 12.5 g/L, sodium acetate: 5.0 g/L and thiourea: 1 mg/L; operating at pH: 4.5–5.0, 90–98 °C for 75–90 minutes (coating thickness 35 ± 5 µm). Continuous job agitation and solution filtration was carried out throughout the plating process. Water rinse
- Heat treatment at 150 ºC for 1 hour.
Gold plating
Gold plating is an extremely important process for engineering applications due to its extreme stability and other unique features that ensures high reliability in adverse environmental conditions. Following are some of the important industrial applications of gold plating.
- To improve the aesthetics and durability due to its outstanding resistance to atmospheric corrosion.
- To increase electrical conductivity: Gold is highly conductive to electricity, only silver and copper are more conductive per volume, but gold has the advantage of anti-tarnishing.
- To enhance the radiation characteristics: Gold is a good reflector of electromagnetic radiation such as infrared and visible light, as well as radio waves. Gold plating is used as protective coatings on many artificial satellites.
- As a solid film lubricant to reduce friction. Because gold has low shear strength, it does not pose a serious threat of cold welding, even in high vacuum conditions [5].
- Protection from atomic oxygen impacts: Gold is one of the most degradation resistant materials with the erosion rate as low as 0.2 ± 0.1 Å per day [6–8].
- As excellent heat radiation surface: Gold plating is used to minimise the radiant heat transfer (radiative coupling) due to inherent very low infrared emissivity (0.02) [9, 10].
Electroless nickel coating on titanium alloy Ti6Al4V provides a very good base for gold plating. Electrodeposition of gold on electroless nickel described above (step 1 to 8) can be followed by using the following steps [4]:
- Activation by dipping in 15 % sulfuric acid (SG 1.83) for 30 seconds. Water rinsing
- Gold strike followed by gold plating in the acid baths as described in Table 1.
The gold coating described herein provides an infrared emissivity as low as 0.02/0.03, and is extremely suitable for harsh space environments. It can withstand extreme temperatures of (-196 ºC to 150 ºC) for an extended period without degradation. The space worthiness of the coating is evaluated by the simulated environmental tests, viz, humidity, thermal cycling, thermo vacuum and thermal stability tests.
|
Bath composition and operating details |
Gold Strike |
Gold Plating |
|
Gold potassium cyanide, [KAu(CN)2], g/L |
3–4 |
10–12 |
|
Citric acid, [C6H8O7.H2O], g/L |
50–60 |
50–60 |
|
Tri sodium citrate, [Na3C6H5O4.2H2O], g/L |
50–60 |
50–60 |
|
pH |
3–5 |
3–5 |
|
Temperature, °C |
60–65 |
68–73 |
|
Current density, A/dm2 |
0.1–0.2 |
0.2–0.4 |
|
Bath agitation and filtration |
Continuous |
Continuous |
|
Time, minutes |
3–5 |
30–40 |
|
Coating thickness, µm |
– |
4–6 µm |
Blackening of electroless nickel
Blackening of electroless nickel alloy coating bestows a pitch black, very high absorptance finish with enhanced wear and corrosion resistance [11–20]. The black finish can be obtained by acid etching of electroless Ni-P coating. During acid etching, the nickel atoms in the nodular Ni-P surface are preferentially removed and some nickel atoms are oxidised. The high absorptance of the coating is associated with unique surface morphology consisting of a dense array of microscopic, conical pores perpendicular to the surface. This structure produced by selective etching of Ni-P acts as light traps and is capable of absorbing 99.5 % light in the solar region (300–2300 nm). The pore diameter, pore depth and pore spacing range from a fraction of micrometre to a few micrometres or about a fraction to several wavelengths of light. Consequently, the pores trap any incident light in a wide spectral range [11–13].
It has been established that compared to high P, a low to medium P, Ni-P alloys impart an electrically conductive deeper black finish with good wear resistance. P content affects the etching behaviour of Ni-P coating. The coating with low P (4 %) etched more completely, with larger crater formation [11–18]. As the P content increases, it becomes more difficult for large Ni-P swaths to be etched; hence, a narrow sharper stalagmite-like morphology is observed with medium P (7 %) coating. However, the Ni-P coating below 6 % P shows poor corrosion resistance without any appreciable improvement in absorptance value. Hence, low to medium P electroless nickel baths operating at a medium acidic pH of 4.5 to 6.0 are recommended. The details of some typical baths are presented in Table 2 [11–14].
|
Bath constituents / Operating conditions |
Bath-1 [11–13] |
Bath-2 [14] |
|
Nickel sulphate hexahydrate, g/L |
30 |
26.3 |
|
Sodium hypophosphite, g/L |
10 |
26.4 |
|
Tri sodium citrate dihydrate, g/L |
12.5 |
– |
|
Sodium acetate, g/L |
5 |
12.3 |
|
Thiourea, mg/L |
1 |
0-2 |
|
D,L-Malic acid, g/L |
– |
4.0 |
|
Citrate acid, g/L |
– |
3.8 |
|
Succinic acid, g/L |
– |
5.9 |
|
Lauryl sodium sulphate, mg/L |
– |
8.0 |
|
pH |
4.5–5.0 |
5.8 |
|
Temperature, °C |
88–90 |
85 ± 2 |
|
Time, minutes |
75–90 |
120 |
|
Plating rate, µm/ hour |
25 ± 5 |
– |
A process of blackening of electroless nickel coating on titanium alloys to produce an ultra-high absorptance coating for space applications was investigated [13]. The electroless nickel coating of 35 ± 5 µm was deposited using the process steps 1–7 for electroless nickel plating of titanium. The blackening of electroless nickel was performed as follows:
- Immersion of Ni-P coated specimen in 9 M nitric acid solution for 30–50 seconds at room temperature.
- After blackening, the test specimens were rinsed quickly and thoroughly with demineralised water.
Effect of concentration of blackening solutions, pH, immersion time, temperature of etching solution and heat treatment was investigated to optimize the blackening process. With an increase in etching time and / or rise in temperature of the etching solution, the nickel content in the coating decreases whereas the phosphorus and oxygen contents increase. This suggests that only nickel atoms in the nodular Ni-P surface are preferentially removed and some of them are converted to nickel oxides (NiO) and Ni2O3) [11–13, 19]. The black colour of coating is associated with its unique crater surface morphology (Fig. 2) combined with the formation of nickel oxides [15–17].
![Fig. 2: SEM of electroless nickel- phosphorus coating after etching [11] Fig. 2: SEM of electroless nickel- phosphorus coating after etching [11]](/images/stories/Abo-2022-11/gt-2022-11-0036.jpg) Fig. 2: SEM of electroless nickel- phosphorus coating after etching [11]
Fig. 2: SEM of electroless nickel- phosphorus coating after etching [11]
Cross section of the blackened electroless nickel coating showed a peak to trough height of 6–7 μm, with a top layer of Ni-P black oxide being ~0.7 μm. When the thickness of this top layer exceeds 1 μm, it often becomes powdery and nonadherent [11]. Heat treatment (190 °C for 2 hour) is recommended to harden the black layer and stabilize the deposit colour. Heat treatment also marginally improves the corrosion and abrasion resistance of the black Ni-P coating [20]. Regardless of heat treatment, black Ni-P coatings show slightly higher corrosion potential compared to unetched Ni-P coatings because of special crack structure.
The space worthiness of the black Ni-P coatings was evaluated by the simulated environmental tests, viz, humidity (relative humidity: 95 ± 5 % at 323 K for 96 hours), thermal cycling (100 cycles, a cycle consists of lowering the temperature to 228 K with a dwell time of 5 minutes and raising the temperature to 358 K with a dwell time of 5 minutes), thermo vacuum performance (10 hot and cold soaks cycles, 228 K to 358 K, in vacuum below 10-5 Torr with a dwell of 2 hours), and corrosion resistance (5 % NaCl at pH 7 for 7 days) tests. The optical properties (solar absorptance and infrared emittance) of the coatings were measured before and after each environmental test to ascertain their stability. The blackened electroless nickel provides high solar absorptance (0.99) and infrared emittance (0.85); has good adhesion, uniformity, and stability in adverse space conditions.
Alhaji, Sadeghi and Kamali [21] studied the effect of pre- and post-heat treatment on the black electroless NiP deposits on Ti6Al4V alloy. Results showed that heat-treatment after blackening causes coating with higher micro-hardness and optical absorption. The electroless nickel process was optimized at temperature: 85–95 °C, and pH 4.3–4.7, and thereafter the blackening was carried out in 9 molar nitric acid for 40 seconds. The blackened electroless nickel after heat-treatment of 4 hours provided high solar absorption of 0.99, which is exceptionally suitable as a solar absorber coating for space and allied applications.
The black ultra-high absorptance coatings are extremely useful to improve the absorptance of thermal detectors and suppress the unwanted reflections or scattered light in optical systems, like telescope housing and baffles where stray light reduction is vital [11, 22–24]. The latter is of significance when it is necessary to reduce the physical size of the instrument whilst not compromising performance. These coatings have been successfully employed in the baffle instruments of many spacecrafts such as Laser Interferometric Gravitational Wave Observatory (LIGO), Cosmic Background Explorer (COBE), Hubble Space Telescope (HST), Extreme Ultraviolet Explorer (EUVE), and Ultra Violet Imaging Telescope (UVIT) of Astrosat. Baffles are frequently built into telescope designs to stop the unwanted light reaching inside telescope tubes / detectors.
Conclusion
Titanium is an ideal metal for a variety of industrial applications because of its high tensile strength to density ratio, ability to withstand moderately high temperatures, and superior corrosion resistance. The major drawback with titanium is its reactivity to atmospheric oxygen. This necessitates a suitable surface modification of titanium before its tangible applications. Autocatalytic nickel-phosphorus coating is widely employed in various industrial applications due to its unique physical and chemical properties, (high microhardness, low porosity, excellent corrosion resistance, low coefficient of friction, etc.). The composition and structure of the coatings can be further tailor designed to suit a variety of industrial applications. Blackening of nickel-phosphorus alloy bestows ultra-high absorptance rich black surfaces with enhanced wear and corrosion resistance. These coatings are extremely suitable for thermal detectors and space telescopes to contain the unsolicited reflections or scattered light in optical systems. Gold coating is extremely important for commercial and technological applications due to its pleasing appearance, very low thermal emissivity, and extreme stability in adverse environmental conditions.
REFERENCES
[1] J.K. Dennis; T.E. Such: Chapter 11, Autocatalytic deposition of nickel in Nickel and Chromium Plating, 3rd Edition, Woodhead Publishing Limited, Cambridge, U.K., (1993), 310–329, doi: 10.1533/9781845698638.310
[2] M. Schlesinger: Chapter 18, Electroless Deposition of Nickel in Modern Electroplating; M. Schlesinger; M. Paunovic (Editors), John Wiley & Sons, Inc.: Hoboken, NJ, 5th Edition, (2014), 447–458, doi: 10.1002/9780470602638.ch18
[3] M.R. Kalantary; K.A. Holbrook; P.B. Wells: Optimisation of a bath for electroless plating and its use for the production of nickel-phosphorus-silicon carbide coatings, Trans. Inst. Met. Finish., 71(1993)2, 55–61, doi: 10.1080/00202967.1993.11870987
[4] A.K. Sharma; H. Bhojaraj: Electroless nickel and gold plating on titanium alloys for space applications, Met. Finish., 90(1992)7, 23–26
[5] A.K. Sharma: Surface engineering for thermal control of spacecraft, Surf. Eng., 21(2005)3, 249–253, doi: 10.1179/174329405X50118
[6] L.S. Morrissey; S.M. Handrigan; S. Nakhla; A. Rahnamoun: Erosion of spacecraft metals due to atomic oxygen: A molecular dynamics simulation, J. Spacecr. Rockets, 56(2019)4, 1231–1236, doi: 10.2514/1.a34414
[7] K.K. de Groh; B.A. Banks; S.K.R. Miller; J.A. Dever: Chapter 28, Degradation of Spacecraft Materials in Handbook of Environmental Degradation of Materials, M. Kutz, (Editor), William Andrew Publishing, (2018), 601–645, doi: 10.1016/b978-0-323-52472-8.00029-0
[8] J. Dever; B. Banks; K. de Groh; S. Miller: Chapter 23, Degradation of Spacecraft Materials in Handbook of Environmental Degradation of Materials, M. Kutz, (Editor), William Andrew Publishing, (2005), 465–501, doi: 10.1016/B978-081551500-5.50025-2
[9] B. Zhang; M. Larson; J. Rodriguez: Passive coolers for pre-cooling of JT loops for deep space infrared imaging applications, Cryogenics, 50(2010)9, 628–632, doi: 10.1016/j.cryogenics.2010.02.019
[10] M. Donabedian: Spacecraft Thermal Control Handbook, Volume II-Cryogenics, Part 2: Cryogenic Radiators and Radiant Coolers, AIAA, The Aerospace Press, El Segundo, California, (2004), 55–90, doi: 10.2514/4.989148
[11] V. Saxena; R. Uma Rani; A.K. Sharma: Studies on ultra high solar absorber black electroless nickel coating on aluminum alloys for space applications, Surf. Coat. Technol., 201(2006)3–4, 855–862, doi: 10.1016/j.surfcoat.2005.12.050
[12] V. Saxena; R.U. Rani; A.K. Sharma: Studies on ultra low reflectance black electroless nickel coating on Invar, Galvanotechnik, 97(2006)4, 827–840
[13] R. Uma Rani; A.K. Sharma; C. Minu; G. Poornima; S. Tejaswi: Studies on black electroless nickel coatings on titanium alloys for spacecraft thermal control applications, J Appl. Electrochem., 40(2010)2, 333–339, doi: 10.1007/s10800-009-9980-5
[14] G.F. Cui; M. Li; D. Li; J. Zheng; Q. Wu: The physical and electrochemical properties of electroless deposited nickel-phosphorus black coatings, Surf. Coat. Technol., 200(2006)24, 6808–6814, doi: 10.1016/j.surfcoat.2005.10.015
[15] S.N. Kumar; L.K. Malhotra; K.L. Chopra: Low cost electroless nickel black coatings for photothermal conversion, Sol. Energy Mater., 3(1980), 519–532, doi: 10.1016/0165-1633(80)90003-9
[16] M. Wierzbicka; A. Malecki: The detailed mechanism of oxidation of Ni-P alloys, J Thermal Anal. Calorim., 55(1999), 981–987, doi: 10.1023/A:1010106506249
[17] A. Malecki; A. Micekilnicka: The kinetics of catalytic nickel deposition from acid-solution, Bull. Polish Acad. Sci. Chem., 43(1995)2, 109–118
[18] C.E. Johnson: Ultra-black coating due to surface morphology, U.S. Patent 4,233,107, (1980); 4,361,630 (1982)
[19] R.J.C. Brown; P.J. Brewer; M.J.T. Milton: The physical and chemical properties of electroless nickel–phosphorus alloys and low reflectance nickel-phosphorus black surfaces, J. Mater. Chem., 12(2002), 2749–2754, doi: 10.1039/B204483h
[20] D. Beckett; Y. Liu; D. Hawthorne: Investigation of the blackening process of electroless nickel-phosphorous coatings and their properties, NASF Annual Conf. 2010 (SUR/FIN 2010), Michigan, USA 14–17, June 2010, 1(2020), 226–238
[21] A. Alhaji; B. Sadeghi; S. Kamali: The effect of pre-heat and post-heat treatment on the hardness and blackening process of electroless {NiP} deposits on titanium alloy, Surf. Topogr.: Metrol. Prop., 8(2020)4, 045031, doi: 10.1088/2051-672x/abd292
[22] S.R. Meier: Methods to suppress stray light in black materials, Proc. SPIE, 5526(2004)1, 195-207. www.deepdyve.com/lp/spie/methods-to-suppress-stray-light-in-black-materialsIcQwccXBwi[23] T. Kralik; D. Katsir: Black surfaces for infrared, aerospace, and cryogenic applications, Proc. SPIE, 7298, Infrared Technology and Applications, XXXV(2009), 729813, doi: 10.1117/12.819277
[24] F. Liu; F. Xing; Z. You: Preparation of Ni-P alloys super-black materials applied to baffle surface, Surf. Eng., 34(2018) 12, 892–899, doi: 10.1080/02670844.2017.1391940