Methanol is not only much easier to transport than hydrogen, but can also be stored almost indefinitely. This means, for example, that green hydrogen could be produced in sunny regions, converted into methanol and transported.
Hydrogen is the beacon of hope for the energy transition: It is intended to make both mobility and industrial processes sustainable. However, there is still a lack of availability at present, as the transportation of hydrogen is expensive. This can be remedied by converting hydrogen into methanol. The carbon dioxide required for methanol production could either be extracted from the atmosphere or come from industrial processes, such as cement production. In addition, methanol offers a very high energy density of around 4.8 kilowatt hours of energy per liter - an order of magnitude higher than that of compressed hydrogen. The International Energy Agency also forecasts very attractive costs for methanol at around six cents per kilowatt hour.
Some challenges still need to be overcome
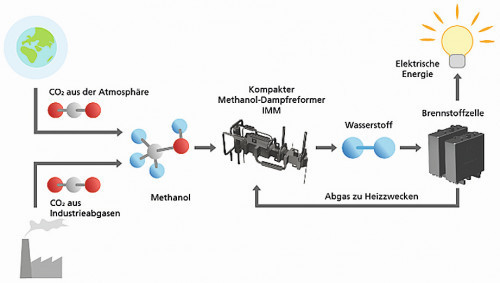 Using methanol as a hydrogen carrier and processing the methanol back into hydrogenIn order touse the energy stored in the methanol, it is converted back into hydrogen and carbon dioxide using a methanol reformer with the addition of water vapor - where the hydrogen is needed, for example in the car. The overall carbon dioxide balance is therefore neutral. However, there are still some problems with conventional reformers. For example, the catalysts required for the reactions. They consist of copper-zinc oxide powder, which is pressed into pellets and poured into the reactor. However, this produces catalyst abrasion, which contaminates the fuel cell. In addition, the catalyst material is not fully utilized and the reaction takes place quite slowly at comparatively low temperatures. Another challenge lies in heat management: heat must be supplied to the reactor to drive the steam reforming reaction - but a great deal of efficiency is lost in the process. The heat from the fuel cell exhaust gas cannot be used efficiently either.
Using methanol as a hydrogen carrier and processing the methanol back into hydrogenIn order touse the energy stored in the methanol, it is converted back into hydrogen and carbon dioxide using a methanol reformer with the addition of water vapor - where the hydrogen is needed, for example in the car. The overall carbon dioxide balance is therefore neutral. However, there are still some problems with conventional reformers. For example, the catalysts required for the reactions. They consist of copper-zinc oxide powder, which is pressed into pellets and poured into the reactor. However, this produces catalyst abrasion, which contaminates the fuel cell. In addition, the catalyst material is not fully utilized and the reaction takes place quite slowly at comparatively low temperatures. Another challenge lies in heat management: heat must be supplied to the reactor to drive the steam reforming reaction - but a great deal of efficiency is lost in the process. The heat from the fuel cell exhaust gas cannot be used efficiently either.
Efficient catalysts, efficient heat management
Researchers at Fraunhofer IMM are therefore developing methanol reformers that overcome these challenges in several publicly funded and industrial projects. For example, the reformer they are developing for mobile applications offers various advantages: For one thing, it requires only one-sixth, or around 17%, of the space taken up by commercially available reformers in the comparable performance class - definitely an important point for mobile applications. The researchers have also optimized the catalyst technology. "We rely on catalyst coatings containing precious metals, which do not cause any abrasion - similar to car catalytic converters," says Dr. Gunther Kolb, Deputy Director of the Institute and Head of Division at Fraunhofer IMM. "Less catalyst material is therefore required. As our catalyst materials also have a higher activity, the required catalyst mass is reduced again, as are the costs." While conventional catalysts increasingly produce by-products such as carbon monoxide during partial load operation - i.e. when the reformer is not working at full capacity - this is not the case with the catalyst from Fraunhofer IMM.
The research team has also optimized the heat management - and therefore the energy efficiency of the reformer: It coats plate heat exchangers with the catalyst material and combines them into stacks of up to 200 plates. When the gas flows over them, it not only comes into contact with the catalyst, but is also heated very efficiently in the small channels. "By using the waste heat, we achieve very good heat integration and high system efficiency," explains Kolb. The researchers also have a possible series production in mind: The reactors can be manufactured in a similar way to high-pressure heat exchangers for motor vehicles, meaning that established processes suitable for mass production can be used.
The researchers are currently creating a prototype with an output of 35 kilowatts, which should be ready by mid-2022. "The project is designed for the longer term, with various prototypes being integrated into land vehicles for testing purposes," says Kolb. For maritime applications, for example, the researchers are developing a reformer with an output of 100 kilowatts. In the long term, it is also conceivable that the reformers, which are currently made of steel, could be produced from lightweight materials such as titanium - an important approach for applications in cars and the like in order to keep weight and therefore energy consumption low.
Photos: Fraunhofer IMM


