In industry, the use of water is essential for production in many areas. In the best case, as with soft water, the conductivity of the city or well water is within the desired range. If the requirements for process water quality are higher, targeted water treatment is essential.
Water treatment
As a rule, city water or well water must be treated before it is used in production. The following processes are available for this purpose:
Water softening
 Fig. 1: Mixed bed ion exchanger In water softening, the water hardness (Ca2+ and Mg2+ ions) is bound to a weakly acidic resin via ion exchange. As Ca and Mg are absorbed, Na+ ions are released in return. The resin bed is regenerated with a saturated sodium salt solution. This means that the regenerate can be discharged directly into the sewer system without further wastewater treatment. The water hardness is reduced to < 0.1 °dH. The salt content and conductivity remain almost constant.
Fig. 1: Mixed bed ion exchanger In water softening, the water hardness (Ca2+ and Mg2+ ions) is bound to a weakly acidic resin via ion exchange. As Ca and Mg are absorbed, Na+ ions are released in return. The resin bed is regenerated with a saturated sodium salt solution. This means that the regenerate can be discharged directly into the sewer system without further wastewater treatment. The water hardness is reduced to < 0.1 °dH. The salt content and conductivity remain almost constant.
The concentrations of iron and manganese are limited to 0.2 mg/l and 0.05 mg/l respectively according to the Drinking Water Ordinance. These concentrations are unproblematic for the resin during water softening. When well water is used, the concentrations of iron and manganese can sometimes be significantly higher than these limits. This then requires the separation of iron and/or manganese before the softening system with additional filter systems.
If soft water is only required for further desalination with reverse osmosis, the use of an antiscalant is also possible as an alternative to the water softener. In this process, antiscalant is dosed directly into the feed to the reverse osmosis in proportion to the volume. The dosing quantity is low and is usually 2-20 ml/m3 deionized water. The pH-neutral antiscalant does not require any further wastewater treatment.
Reverse osmosis
 Fig. 2: Polisher After the water has been softened in a first step, the reverse osmosis process can be used for salt separation and thus for reducing conductivity. In this process, the salts dissolved in the water are separated by increasing the pressure via a semi-permeable membrane. Depending on the system design, the conductivity is reduced to 0.5-3.0 % of the conductivity in the water supply.
Fig. 2: Polisher After the water has been softened in a first step, the reverse osmosis process can be used for salt separation and thus for reducing conductivity. In this process, the salts dissolved in the water are separated by increasing the pressure via a semi-permeable membrane. Depending on the system design, the conductivity is reduced to 0.5-3.0 % of the conductivity in the water supply.
The ions from the raw water are then concentrated in the resulting concentrate (approx. 10-25 % of the inflow volume). It is therefore usually possible to discharge the concentrate into the sewer system without treatment.
A further reduction in conductivity (e.g. to < 1 µS/cm) requires post-treatment of the permeate from the reverse osmosis. The usual processes for this are
- Mixed bed ion exchanger
- Electro-deionization (EDI)
- Polisher (disposable resins)
Depending on the specifications and system design, we then achieve an electrical conductivity of up to 0.06 µS/cm at a water temperature of 25 °C (Fig. 1 and 2).
Ion exchanger
As an alternative to reverse osmosis, an ion exchanger can be used to adjust the water hardness and conductivity of the process water to the desired quality in just one step. This also applies to the separation of iron and manganese if well water is used. The ion exchange process is the separation of the interfering ions dissolved in the water by means of ion exchange, through the exchange of hydrogen ions and hydroxide ions.
The regeneration of the loaded resin is usually carried out with a diluted hydrochloric acid and a diluted sodium hydroxide solution. The following resin combinations are common:
- KA + aA → conductivity < 30.0 µS/cm
- KA + aA + AA → conductivity < 10.0 µS/cm
- KA + aA + AA + KA → Conductivity < 2.0 µS/cm
- KA + aA + AA + MB → Conductivity < 0.2 µS/cm
Meaning:
KA: strongly acidic cation exchanger
aA: weakly basic anion exchanger
AA: strongly basic anion exchanger
MB: mixed bed exchanger
With a demand for demineralized water of more than 100m3 per day and raw water with a hydrogen carbonate concentration (HCO3) greater than 300 mg/l, the use of a trickling tower results in a significant reduction (> 50 %) in caustic soda.
As acids and alkalis are used during regeneration, the regenerate from an ion exchanger system must be neutralized in a wastewater pre-treatment system and then discharged into the sewer via a final pH control.
Rinsing water circulation
The aim of the rinse water circuit is to reduce the amount of waste water produced to a minimum for a given product throughput while maintaining the required rinse quality.
The following points must be taken into account when planning an ion exchanger circulation system. The first step in calculating rinse water volumes is to determine the expected carryover. The carryover depends on many factors, such as
- Type of construction: rack or drum system
- Planned throughput of goods inm2/h or kg/h
- Type of product, e.g. smooth surface, with structure or scooping parts
- Viscosity of the process bath
- Dwell time after removal from the process or rinsing bath (draining times)
- Additional measures: squeezing, blowing off with compressed air, e.g. internal rinsing of drums
Taking into account the points listed above, the carry-over is in the range of 0.05-0.50 l/m2 or 0.5-5.0 l/50 kg. Higher values are also possible for scooping parts.
The second factor for calculating the rinsing water quantity is the rinsing criterion required for product quality, which represents the dilution of the process bath by the rinsing process.
Rinsing criterion = process bath concentration / rinsing bath concentration - usual values:
- Degreasing: 500-1000
- Pickling: 1,000-2,000
- Metallizing: 2,000-5,000
- Metallizing (cyanide): 10,000
- Hart-/Glanzchrom: 10.000–30.000
- Electronics/optics: sometimes > 100,000
These rinsing techniques are used in most cases:
- Pedestal sink
- Cascade sink
- Combination pedestal sink - recirculation sink
- Combination cascade sink - recirculating pre-clean sink
- Circulation of hot rinsing water
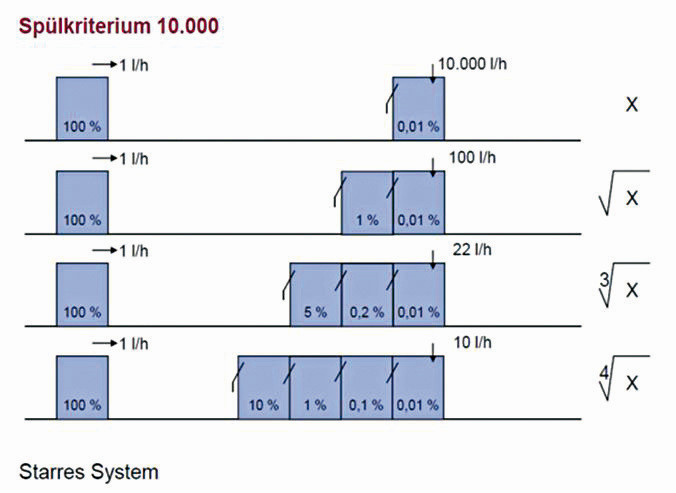
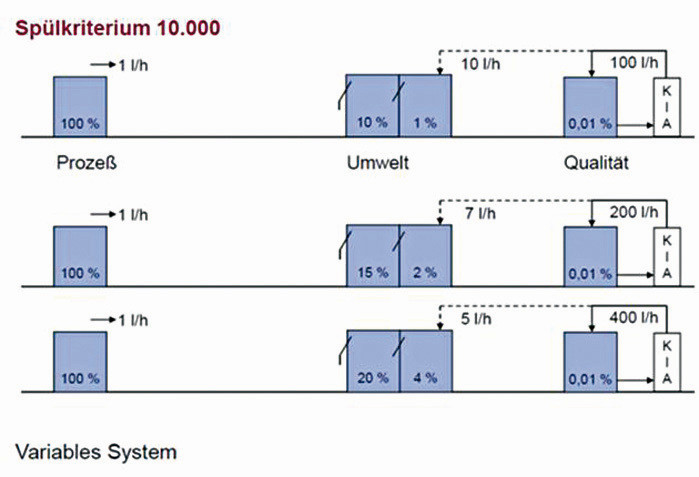
Figure 3 shows the influence of the number of rinsing stages on the rinse water volume. Figure 4 illustrates the flexibility of using an ion exchanger system for rinse water recirculation. Depending on the selected rinse water volume in the pre-rinse cascade, the volume of waste water produced and therefore the loading of the ion exchanger system changes. With a pre-rinse criterion of 100, for example, only 1 % of the discharged load reaches the ion exchanger system.
Pre-rinsing criteria of 10-100 are generally used, depending on the concentration of the process bath. The graph above shows examples of pre-rinse criteria of 100, 49 and 25.
The continuous discharge of a partial quantity into the pre-rinse cascade also limits the increase in organics in the rinse water circuit.
Conclusion:
Which of the processes described above is used for the production of demineralized water at the respective site depends on many factors.
Process-neutral advice from a specialist company is a good way to go. With the use of an ion exchanger circulation system, a minimum amount of wastewater can be achieved with a very high rinsing quality.
In recent years, recirculation systems designed for only one or a maximum of two technological rinsing stages have been increasingly used. The sinks where the operation of recirculation systems makes economic and ecological sense should be decided together with a specialist company or a specialist consultant.