Silver contact coatings are widely used in the automotive sector due to their good electrical conductivity and corrosion resistance, but are problematic due to cold welding and a high coefficient of friction. New requirements, for example in electromobility, demand silver variants that can achieve 10,000 mating cycles and more without any loss of properties. These high-performance silver variants include hardened silver alloys and dispersion coatings with solid-state lubricants. Wear tests show that hardness and wear resistance are not the same for hardened silver alloys and that dispersion coatings offer improved friction properties.
In the automotive sector today, silver is mainly used as a contact coating alongside tin and hard gold [1]. Due to the very good electrical conductivity of silver, it is particularly suitable for high-current and high-temperature applications. Automotive trends such as the electrification of the powertrain, miniaturization and lightweight construction are continuously increasing the demands on connection technology. While the design of the vehicle electrical system is challenging but easy to define, the charging interface is quite different: This is a connection between the publicly accessible infrastructure and the vehicle, which is regularly disconnected, resulting in demands for 10,000 and more mating cycles. In order to be able to use high currents of up to 800 A for fast charging of the vehicle in the future, a high current carrying capacity is required. The combination of frictional wear due to a high number of mating cycles with possibly contaminated and damaged contacts in the charging gun and high charging currents means a significant thermal risk over the required service life.
Silver and silver-based contact coatings are extremely well suited to meeting these requirements. Pure silver coatings are characterized by [2]:
- low contact resistances, which result from the advantageous combination of a low specific electrical resistance ρ = 16 mΩ mm2 m-1 and a medium contact hardness
- good temperature stability up to 140 °C without nickel interlayer and up to 160 °C with nickel interlayer
- Good corrosion resistance under automotive conditions
- low costs compared to precious metal coatings based on gold/palladium
- However, pure silver coatings also have some functional disadvantages [2]:
- a tendency to tarnish in sulfur-containing environments and therefore the need to use passivation
- relatively high coefficients of friction µ = 0.8-1.2
- high frictional wear with mating cycles >50
- the tendency to cold welding, especially at high surface pressures and high temperatures
The characteristics of the favorable and unfavorable properties depend on the electrolyte used, the electrolyte base and the additives. The unfavorable properties limit the use of silver coatings in new fields of application. For this reason, extensive efforts are being made to improve the properties of silver as a contact material [1], [3]. These are aimed in particular at the friction and wear properties, temperature stability and corrosion resistance, whereby different properties must be optimized depending on the load in the application. For example, a low coefficient of friction is advantageous for low insertion forces, but reduces the holding forces in vibration applications. Various approaches are being pursued, such as increasing the contact hardness, incorporating lubricants, improving the surface structure and preventing diffusion and recrystallization effects.
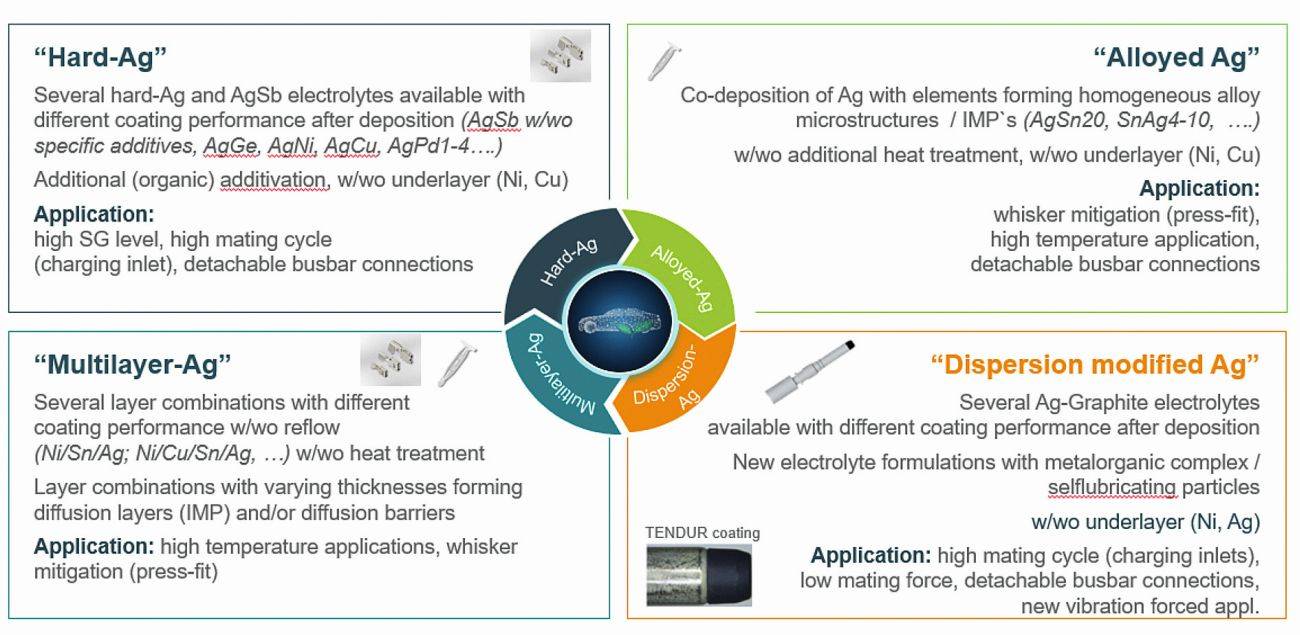 Fig. 1: Overview of the modification options for silver coatings with examples
Fig. 1: Overview of the modification options for silver coatings with examples
Electroplated coatings can be changed by modifying the deposition parameters, adding organic coating components, alloying with other elements or co-depositing particles (Fig. 1). In doing so, the fundamentally good properties of silver contact layers should be retained. This limits the scope, as certain elements and the addition of certain elements or excessive concentrations of elements greatly reduce the electrical conductivity.
Differentiating the performance of layer systems
Before contact layers are tested in products for special applications, they should be thoroughly analyzed. This can be done using a model system that allows comparability regardless of the geometry and properties of the connector design. So-called flat cap samples, where one contact side is flat and the mating contact has a shaped spherical cap (R = 5 mm, R = 4 mm), simulate a simple spring/blade combination. These patterns can be coated both in laboratory processes (beaker) and in series processes (rack, barrel or strip electroplating) (Fig. 2).
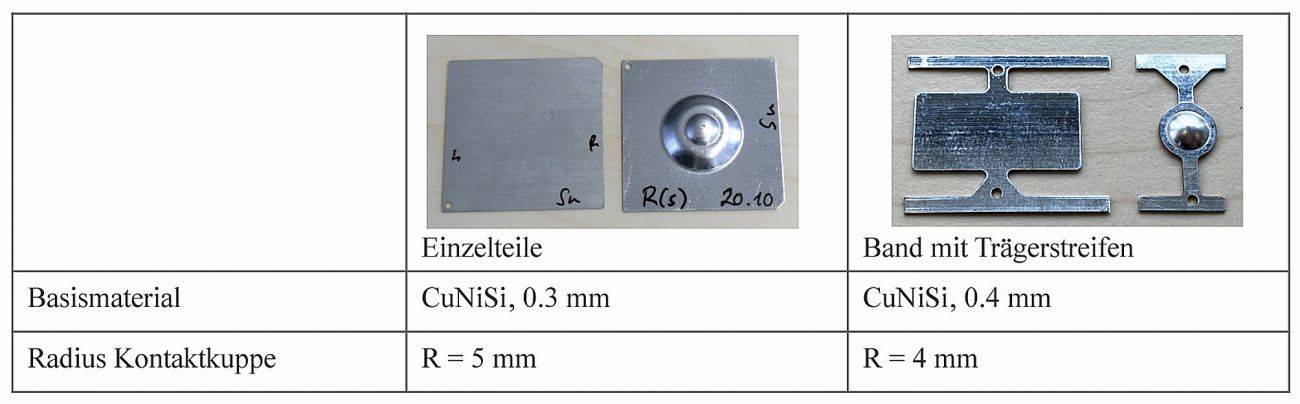 Fig. 2: Geometry of the contact patterns
Fig. 2: Geometry of the contact patterns
The most important functional coating properties, such as contact resistance, friction coefficient µ = FR/FN (FR: frictional force, FN: contact normal force) and frictional wear/wear resistance are determined using suitable contact test stations [4], [5]. In addition, microscopic material properties such as roughness, structure, chemical composition and hardness can be determined using analytical methods such as confocal microscopy, scanning electron microscopy (SEM), energy dispersive X-ray analysis (EDS), focused ion beam microscopy (FIB), nanoindentation and others.
Superimpositions of certain influences from applications such as corrosion and ageing effects are usually investigated directly on the contact/product with storage under different conditions, based on corresponding automotive standards [6]:
- Storage in dry heat at 105/150/180 °C, 1000 h
- Storage in harmful gas, 21 days (multi-component climate: 0.2 ppm SO2, 0.01 ppm H2S, 0.2 ppm NO2, 0.01 ppm Cl2, 25 °C, 75 % relative humidity)
- Pushing and pulling after/under atmospheric exposure such as salt & sand
- Vibration profiles
In addition to the automotive test standards TLF 0214 and 0215, there are also OEM-specific standards, which do not allow a general statement to be made on the suitability of surfaces for the various applications due to the different severity of the loads.
Contact resistances and coefficients of friction
A contact coating must ensure that the contact resistance is as low as possible over the service life of the connector, thereby minimizing current heating. The second focus is on the coefficient of friction, which influences the insertion and withdrawal forces as well as wear. As possible frictional wear can also influence the contact resistance, the contact resistance is measured on the samples as a function of the normal contact force on the one hand and the contact resistance together with the coefficient of friction during a cyclical tribological load on the other.
Hard silver coatings
A wide variety of hard silver electrolytes are available on the market today, some of which differ greatly in terms of their contact-specific properties despite their identical nominal composition (Fig. 3).
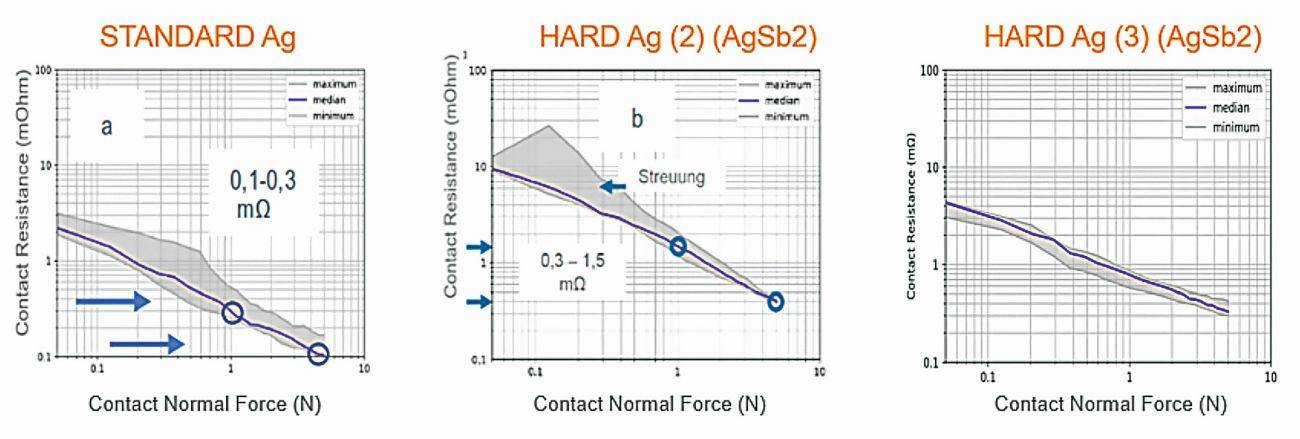 Fig. 3: Contact resistance curve of two hard-Ag variants with the same chemical composition compared to a pure-Ag coating with the same layer thickness and identical layer structure
Fig. 3: Contact resistance curve of two hard-Ag variants with the same chemical composition compared to a pure-Ag coating with the same layer thickness and identical layer structure
Experience has shown that differences in the contact resistance level in the relevant normal contact force range of 1-5 N even increase after several mating cycles (Fig. 4). The contact pairing is also decisive here: is it mated against a similar surface (vs. like) or against a different surface such as pure Ag. Unfortunately, the pairing for use in the field cannot always be determined, as the contact parts may be manufactured by different suppliers. This must therefore be taken into account in the overall assessment.
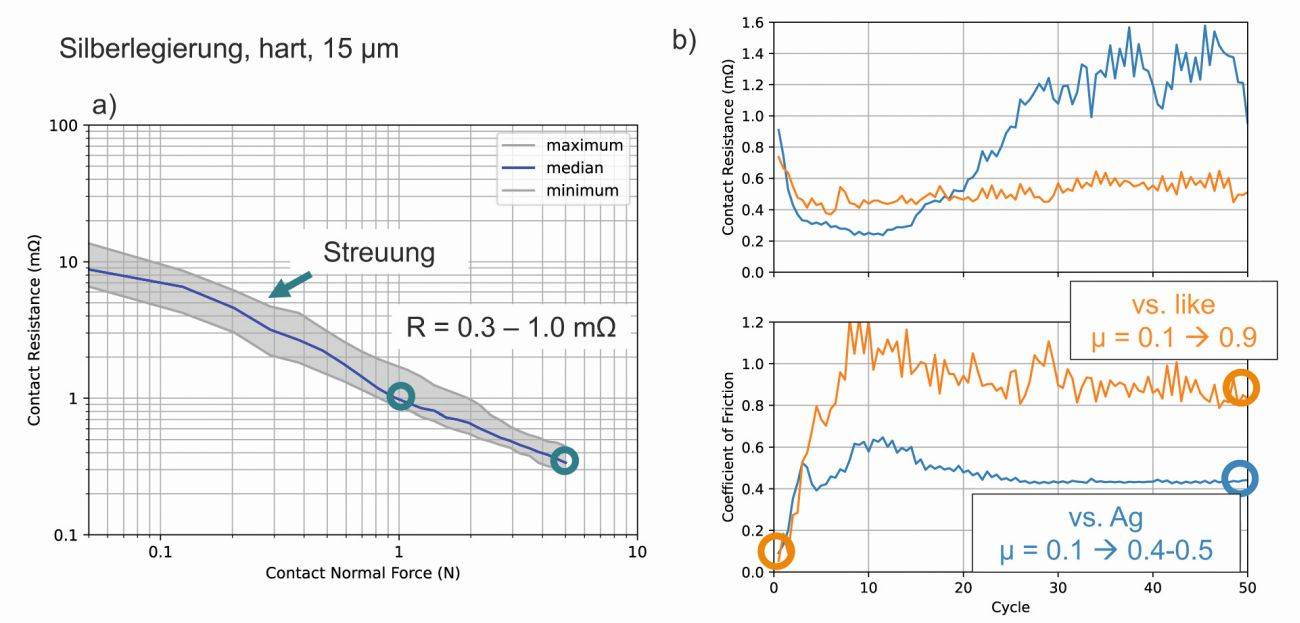 Fig. 4: Contact resistance and friction coefficient of a hard AgSb alloy with 150 HV, measured in the initial state and after 50 friction cycles, against a contact surface of the same type and against pure Ag (contact normal force 2N)
Fig. 4: Contact resistance and friction coefficient of a hard AgSb alloy with 150 HV, measured in the initial state and after 50 friction cycles, against a contact surface of the same type and against pure Ag (contact normal force 2N)
In the example shown here, the coefficient of friction increases from an initial 0.1 to over 1.0 during the first 10 cycles and then remains at a high level until the 50th cycle. When the coating is paired with a standard silver-coated dome, the coefficient of friction is also initially 0.1, but only rises to 0.4-0.6. The contact resistances of both pairings are initially comparable, but after about 20 cycles the contact resistance increases more with unequal pairing than with equal pairing. This may be an indication of incipient frictional wear.
The risk of the behaviour shown in Figure 4 with simultaneous wear can be minimized by improving the tribological behaviour.
As a second example, Figure 5 shows comparable measurements of a friction-reducing hard silver alloy. The contact resistances are in the range of 0.3-1.1 mΩ at FN = 1-5 N. When mating with identical contact surfaces, the coefficient of friction is initially 0.2 and increases to 1.1 after 50 cycles. When paired with a standard silver-coated tip, the coefficient of friction is initially 0.3, but only rises to 0.6 after 50 cycles; the frictional wear is relatively low due to the lubricating organic additives.
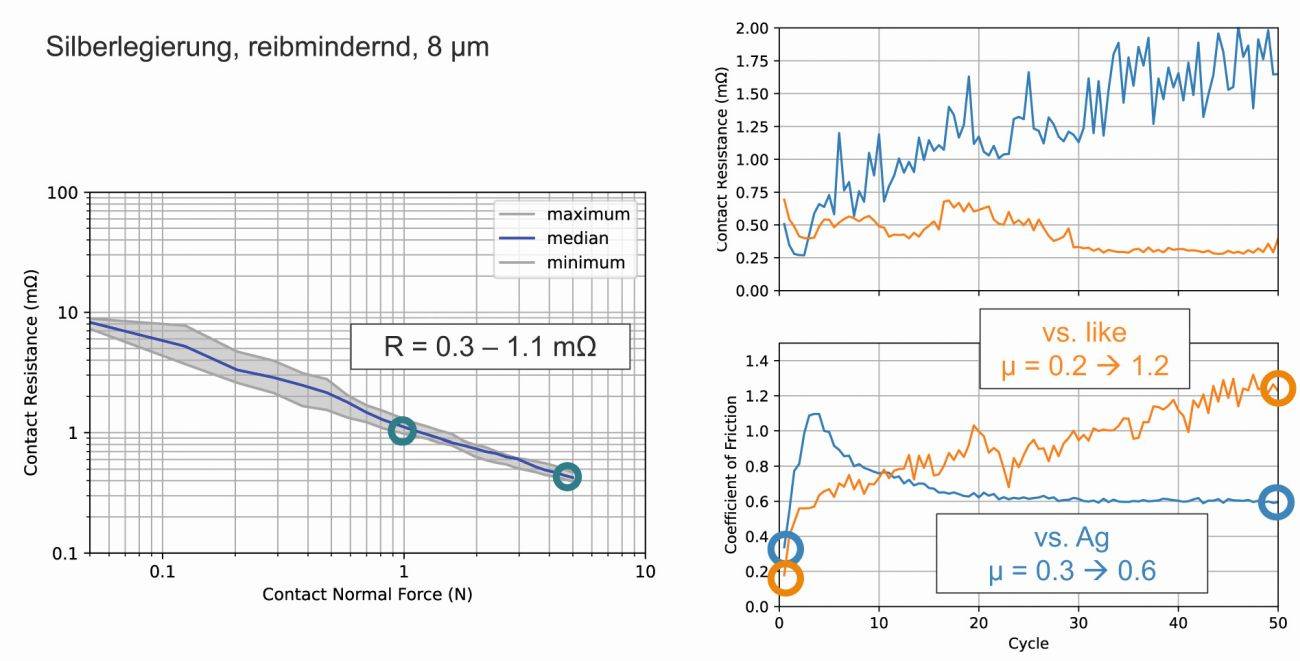 Fig. 5: Contact resistance and friction coefficient of a friction-reducing hard AgSb alloy with 170 HV, measured in the initial state and after 50 friction cycles, against a similar contact surface and against pure Ag (contact normal force 2N)
Fig. 5: Contact resistance and friction coefficient of a friction-reducing hard AgSb alloy with 170 HV, measured in the initial state and after 50 friction cycles, against a similar contact surface and against pure Ag (contact normal force 2N)
The two examples clearly show that the performance of a silver alloy coating is not defined solely by its nominal composition and hardness, but requires further descriptive features or the naming of the brand name of the electrolyte used, for example.
Silver dispersion coatings
Electrolytes available on the market for Ag dispersion coating have so far mainly concentrated on silver with graphite or diamond particles, which are co-deposited and thus incorporated into the silver matrix.
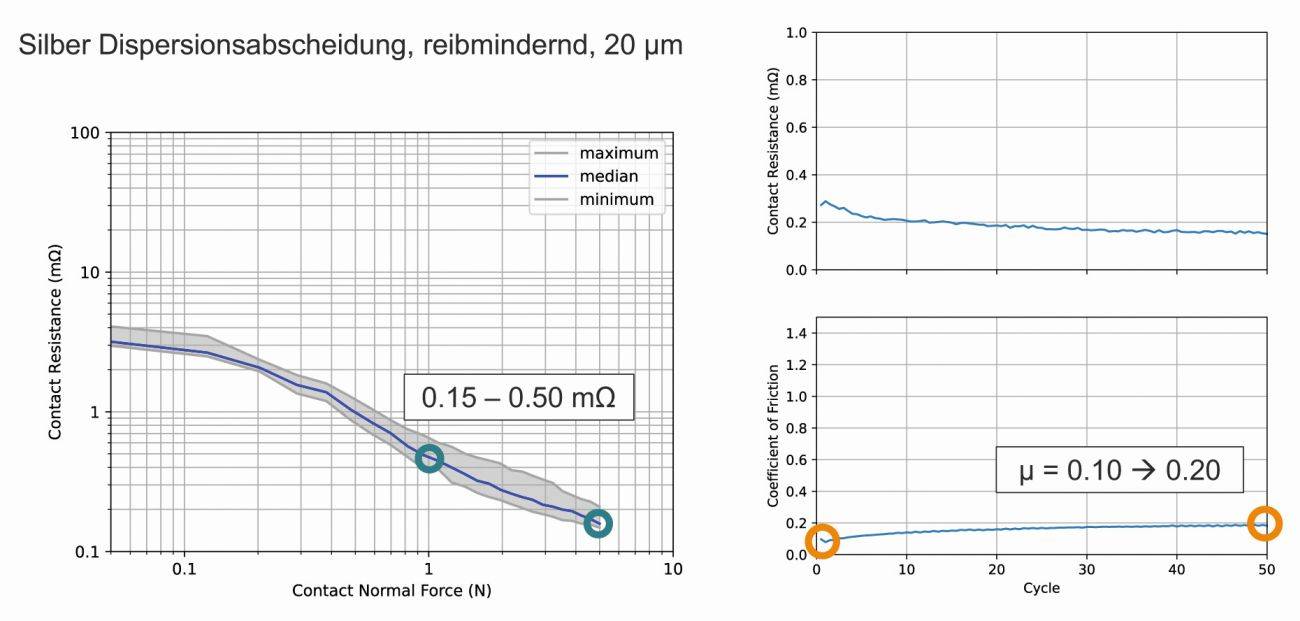 Fig. 6: Contact resistance and friction coefficient of a silver graphite coating with 105 HV, measured in the initial state and after 50 friction cycles, against a similar contact surface (contact normal force 2N)
Fig. 6: Contact resistance and friction coefficient of a silver graphite coating with 105 HV, measured in the initial state and after 50 friction cycles, against a similar contact surface (contact normal force 2N)
Figure 6 shows the measured values of a friction-reducing dispersion deposition with soft graphite particles in a silver matrix. The graphite particles incorporated in the layer (Fig. 7) lubricate the contact surface and lead to lower friction. At 0.15-0.50 mΩ, the contact resistance at FN = 1-5 N is lower than with AgSb silver alloys. This can be explained both by the lower hardness of the layer (80-100 HV) and by the good resistivity of the unalloyed silver matrix.
 Fig. 7: FIB sections through the various silver layers investigated
Fig. 7: FIB sections through the various silver layers investigated
When pairing the same coatings on both samples, the friction coefficients of this coating are low, ranging from 0.1-0.2 over the 50 cycles. The contact resistances during the friction cycles are stable at values around 0.2 mΩ over the 50 cycles. There is no significant difference between the same pairing and pairing with a standard silver-coated tip.
An Ag electrolyte is now available on the market, which produces a friction-reducing coating through the co-deposition of organic particles. Similar to the Ag-graphite surfaces, the friction coefficient is at a low level, in the range µ = 0.2 - 0.3 (Fig. 8). Due to the organic additives, the contact resistances are slightly higher than for the silver graphite coating (Fig. 6), but lower than for hard silver coatings (Fig. 3).
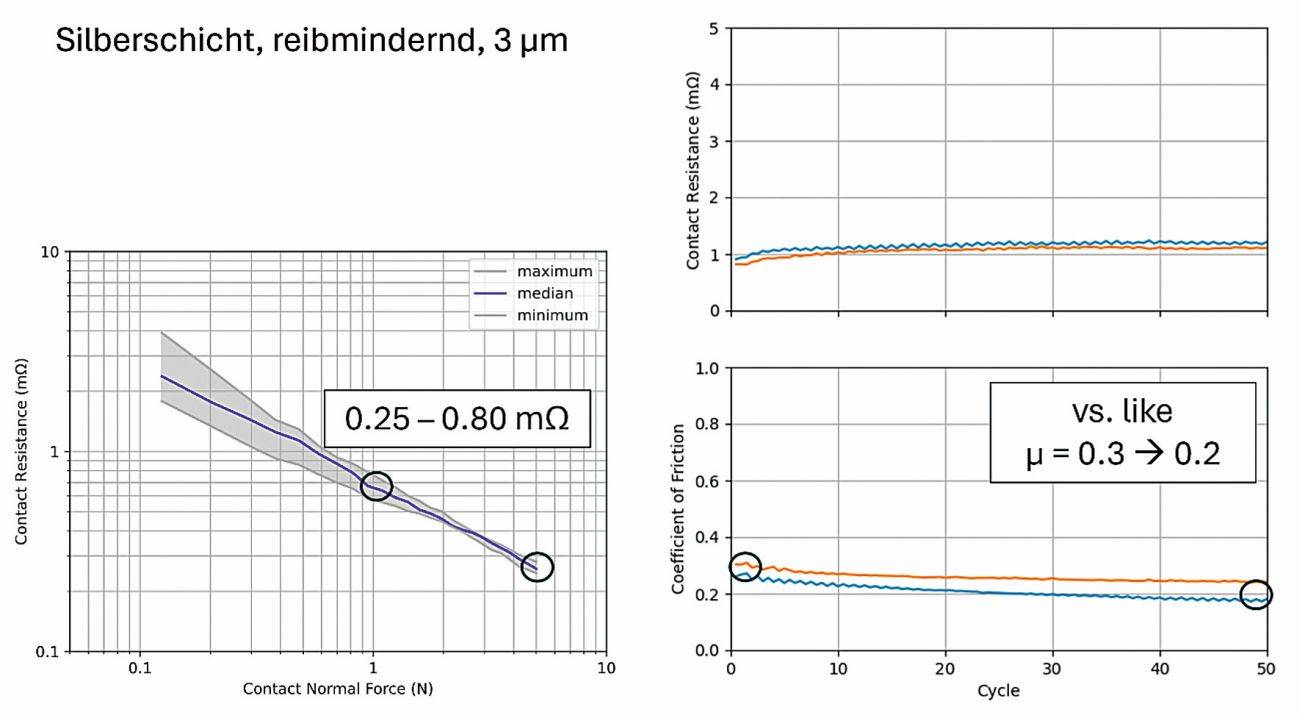 Fig. 8: Contact resistance and friction coefficient of a friction-reducing silver coating in the initial state and after 50 friction cycles measured against a similar contact surface (contact normal force 2N)
Fig. 8: Contact resistance and friction coefficient of a friction-reducing silver coating in the initial state and after 50 friction cycles measured against a similar contact surface (contact normal force 2N)
The different behavior of the silver variants in terms of contact resistance and friction coefficient is due to the chemical composition and microstructure of the layers. The microstructure of the silver variants was investigated using focused ion beam (FIB) sections. Sections of the layers in their initial state are shown in Figure 7. The layers have a clearly different morphology: the particles incorporated into the layer are clearly visible in the dispersion layers. The silver alloys, on the other hand, show a fine crystalline, homogeneous structure, which should remain stable over the service life in order to maintain the desired properties. Increased application temperatures can lead to recrystallization or segregation/segregation under certain circumstances.
Transferring the laboratory results to the product
The laboratory sample geometry simulates a simple spring/knife contact, which allows a neutral comparison of different coatings. The design influence is ignored. Using the example of a charging socket connector in a pin-tulip design, a hard Ag surface and an Ag-graphite surface were tested at product level in comparison with pure Ag. The most important properties for this application are the development of the coefficient of friction/wear of the surface and, in parallel, the contact resistance over at least 10,000 mating cycles. At flat cap level, the differences between a hard AgSb2 coating and the silver graphite coating are obvious:
Flat & cap test: silver graphite dispersion coating, friction-reducing
- In the range FN = 1-4 N, the coefficient of friction is constant at 0.2 over 10,000 cycles, the resistance is in the order of 1 mΩ.
- At FN = 0.5 N, the coefficient of friction µ < 0.2, but the contact resistance is significantly higher. This is an indication that the contact is floating on the lubricating layer created by the graphite.
- At FN = 6 N, the coefficient of friction is initially µ = 0.2, but after approx. 500 cycles it increases, a sign of higher wear
Flat & cap test: silver alloy AgSb2, hard
- The contact resistances of the hard silver alloy increase significantly after just a few cycles, at forces FN > 4 N they drop again to approx. 1 mΩ, at lower forces they remain permanently high.
- The coefficients of friction are clearly dependent on the normal contact force and range between 0.3 and 0.8.
- For FN > 0.5 N, significant wear is already present after 2000 cycles.
Wear rates can be determined from the depth of the wear track determined using confocal microscopy. At FN = 2 N, they are (0.4 ± 0.1) µm/1000 cycles for the friction-reducing silver dispersion layer and (3.1 ± 0.9) µm/1000 cycles for the hard silver alloy. In general, these wear rates increase with the contact normal force.
The coatings show through-wear when the entire contact coating is removed by the frictional wear and the base material is exposed. This condition is not acceptable in applications, as the corrosion of the exposed base materials can lead to undefined increases in contact resistance over the service life.
Tulip & Pin product test
Mating cycle tests under field conditions on a real pin-socket connection show that pure Ag and hard Ag surfaces are worn through as a pin surface after just 2,000 mating cycles, depending on the layer thickness (Fig. 9). Experience from many mating cycle tests confirms the statement that with Ag-based coatings, hard does not mean more wear-resistant; even coating thicknesses in the range (40...60) µm show signs of wear with hard Ag surfaces, which have a negative effect on the electrical contact behavior. High coating thicknesses are also associated with higher costs.
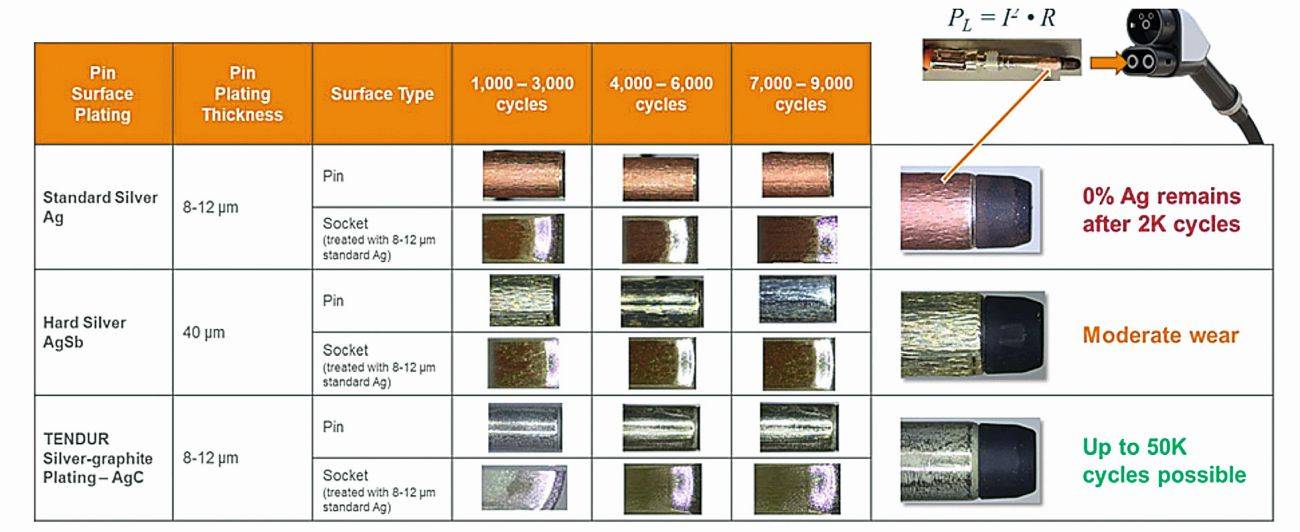 Fig. 9: Surface condition of various coatings after 2,000...9,000 mating cycles of a CCS2 8 mm DC pin surface mated against a tulip contact, coated with pure Ag
Fig. 9: Surface condition of various coatings after 2,000...9,000 mating cycles of a CCS2 8 mm DC pin surface mated against a tulip contact, coated with pure Ag
The approach of positively influencing wear processes by incorporating self-lubricating particles such as graphite into the silver coating is already clearly visible: the surface shows no change in color after thousands of mating cycles, which is a good indicator that the coating has not worn through. The graphite particles form a nm-thick graphite film in the friction and contact zone, which has the effect of a solid lubricant. This and thus the self-lubricating properties of the graphite particles also act as protection for the opposite side if only one partner of a loading connection is coated with this surface. The wear condition of the contact surface is directly linked to the contact resistance behavior. Wear on one contact partner also causes further wear on the other side over the service life, which leads to a change in the contact resistance behavior over time. Field measurements have shown that the distribution curve of the measured contact resistances in the charging path shifts towards higher values as the service life increases (Fig. 10). The probability of higher resistance values occurring increases significantly with Ag and hard Ag surfaces compared to the silver graphite coating. During the charging process, this means that as the number of charging processes increases, the risk increases that the charging current will be reduced during the DC charging process due to the T-rise specification + max. 50 K, thus prolonging the charging process [7].
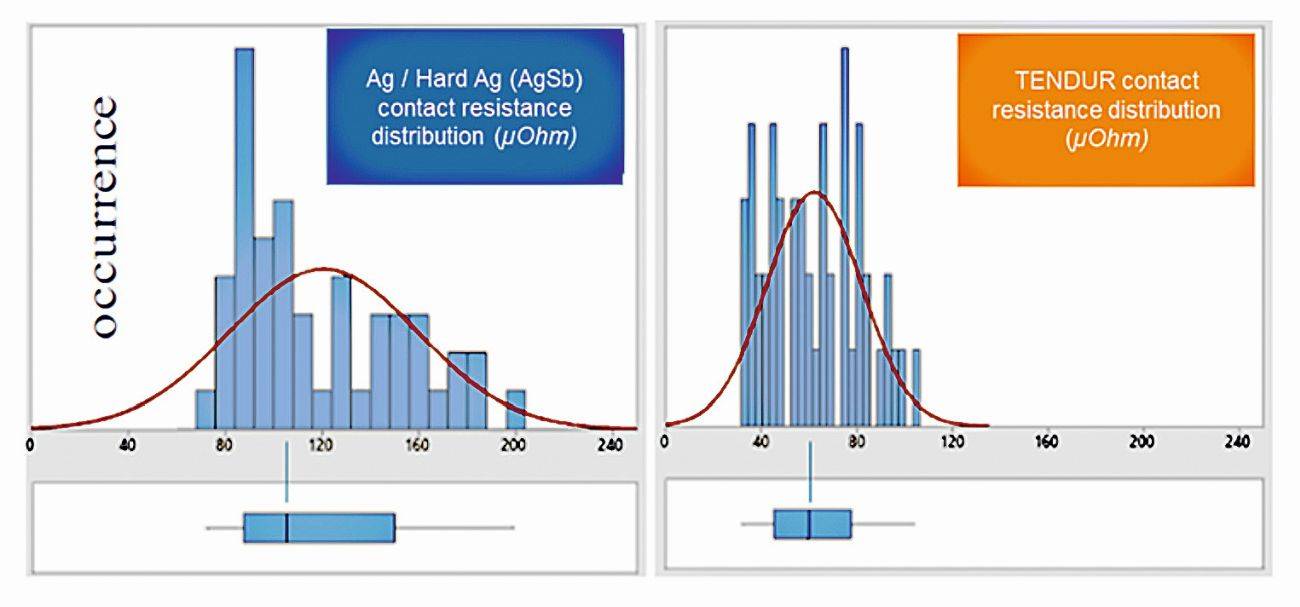 Fig. 10: Exemplary distribution of contact resistance values and the probability of higher values occurring for pure Ag, hard AgSb2 and TENDUR silver graphite contact surfaces in the inlet, measured after 2,000...9,000 mating cycles - contact coating on the infrastructure side pure Ag
Fig. 10: Exemplary distribution of contact resistance values and the probability of higher values occurring for pure Ag, hard AgSb2 and TENDUR silver graphite contact surfaces in the inlet, measured after 2,000...9,000 mating cycles - contact coating on the infrastructure side pure Ag
Summary and outlook
Silver and silver-based coatings are very well suited to the new technical challenges facing connectors for automotive applications with high mating cycles or high application temperatures. This applies in particular to the field of electromobility, where high power and currents have to be transmitted. However, the wear properties and temperature stability of pure silver alone are not sufficient for a large number of applications, which is why many new silver variants are being developed. These variants differ significantly from each other and from pure silver.
- Wear properties can be improved by self-lubricating/friction-reducing layers.
- Greater hardness of a layer does not mean greater wear resistance. In addition, the contact resistance of harder coatings is higher than that of softer coatings. This can be a disadvantage, especially when used for the transmission of high power.
- Certain silver variants exhibit significantly different wear behavior in paired combinations compared to non-paired combinations.
- Silver dispersion layers with solid lubricant (silver-graphite) have the advantage of low contact resistance (soft silver matrix) in combination with low wear due to the "built-in" lubrication [7].
- Wear properties generally differ with regard to the load. For example, it can be seen that lubrication is particularly important with many mating cycles or frictional wear. On the other hand, lubrication can be disadvantageous in the case of vibration loads, as holding forces between the contacts are important and flexing rather than frictional movements occur.
- Hard silver coatings do not necessarily have identical properties, even if they have the same composition.
Overall, high-performance silver variants have the potential to open up new fields of application and overcome some of the disadvantages of pure silver coatings.
Literature
[1] H. Endres, Practical handbook on connectors. Vogel book publishing house, 2021.
[2] M. Myers, "The Performance Implications of Silver as a Contact Finish in Traditionally Gold Finished Contact Applications," in Proceedings of the 55th IEEE Holm Conference on Electrical Contacts, 2009, pp. 310-318.
[3] M. Braunovic', "Electrical Contacts: Fundamentals, Applications and Technology," 2006.
[4] S. Sachs, H. Schmidt, A. Bäumer, S. Thoss, A. Jeblick, and M. Schecker, "Systematic and efficient characterization of the contact physics of connectors with a newly developed measuring apparatus," in Electrical and Optical Interconnection Technology 2013, Lemgo, Germany, 2013.
[5] S. Sachs and H. Schmidt, "Investigation of local contact resistances by scanning probe micro-scanning of contact surfaces," in Electrical and Optical Interconnection Technology 2021, Lemgo, Germany, 2021.
[6] "Technical guideline - TLF 0214, Validation of automotive low-voltage connectors." ZVEI - German Electrical and Electronic Manufacturers' Association, 2020.
[7] M. Oberst, I. Buresch, and M. Ludwig, "Langzeitstabile Hochstrom-Verbindungen im elektrischen Ladepfad"; in 16. Anwenderkongress Steckverbinder, Würzburg, 2022, Elektronikpraxis.


