The story of Columbus' egg is well known, although he certainly had to be careful that it didn't leak. But perhaps it was also hard-boiled. Magicians live from the fact that it is easier to trick people into believing something magical than to make them look for the simple and natural process.
 Fig. 1: 2-ruble Solzhenitsyn commemorative coinWhenthe scantily clad lady disappears from the cage, innocent people do not immediately suspect a trapdoor.
Fig. 1: 2-ruble Solzhenitsyn commemorative coinWhenthe scantily clad lady disappears from the cage, innocent people do not immediately suspect a trapdoor.
After all, there are still scientists who are not taken aback - such as Conrad Röntgen[2], who did not believe in a ghost when he saw the bones of his wife's hand. Process engineers, on the other hand, often suspect the hand of fate behind the disappearance of metal coatings during soldering - after all, it was quite shiny before the process.
Soldering is a chemical-physical process in which joints are formed between metals. The most important and largest metal, tin, can be described as the 'aggressor', as most of the reactions take place with this metal while the others are more or less uninvolved in the solder. Tin has a much higher proportion in the newer alloys than in the lead-containing solders used in the past. For this reason, and also because of the higher temperatures, alloying occurs more frequently.
When studying the influence of tin on the various metals, it is important to consider not only the tin content and the temperature, but also the amount of solder present and the type of supply. If the solder is moving, as in wave soldering, the situation is different from a static bath, because the moving solder neither loses temperature in the vicinity of the immersed metal nor does it become enriched with deposited atoms and molecules. The reactions slow down the lower the temperature and the more of the deposited metal is present in the tin, until a certain saturation is reached.
This is how wave soldering with an 'infinite' supply of solder differs from selective soldering - especially if it is an immersion process - which has a limited quantity and reflow soldering, where there is only an extremely small supply of paste (and possibly a molded part).
It goes without saying that different temperatures and different times are used in each case via liquidus - every process engineer knows that.
So it is not hocus-pocus if the tempering of a component suddenly disappears, but a completely normal process that allows many conclusions to be drawn. Anyone who is self-critical will first think of incorrect process parameters or a lack of control and monitoring of the machines.
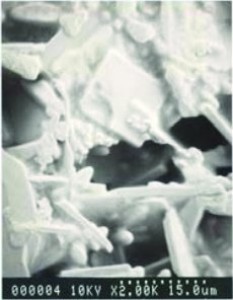 Fig. 2: Scanning electron micrographs of the brittle AuSn4 diffusion zoneButit is by no means unlikely that the component is to blame. In this case, your purchasing department has ordered cheap goods again in order to get a good Christmas bonus from management and quality control has been sloppy or has no idea how to properly test and evaluate the solderability of components - or you didn't have the guts to report the poor quality to the top. This overlooks the fact that the costs for a repair after soldering are far higher than the few extra pennies for proper components.
Fig. 2: Scanning electron micrographs of the brittle AuSn4 diffusion zoneButit is by no means unlikely that the component is to blame. In this case, your purchasing department has ordered cheap goods again in order to get a good Christmas bonus from management and quality control has been sloppy or has no idea how to properly test and evaluate the solderability of components - or you didn't have the guts to report the poor quality to the top. This overlooks the fact that the costs for a repair after soldering are far higher than the few extra pennies for proper components.
The dissolving and dissolving of metals in the solder - it is well known that occasionally very thin connecting wires simply disappear during wave soldering - also has a downside. The metal has to go somewhere and it is not uncommon to find it in the solder joint. There it then has an influence on the longevity of the connection, whereby the different types of stress such as impact, bending or shearing must be assessed differently. The influence of gold in the solder joint has been known since time immemorial thanks to warnings from the IPC[3] and their standards also specify certain limit values that should be adhered to. Gold embrittlement in solder joints is evident if the percentage ratio of the weight of the gold to the weight of the solder is more than 3 % of the total weight of the solder alloy. Recent studies indicate that problems can occur even at lower values.
Less is known about other impurities such as silver etc.
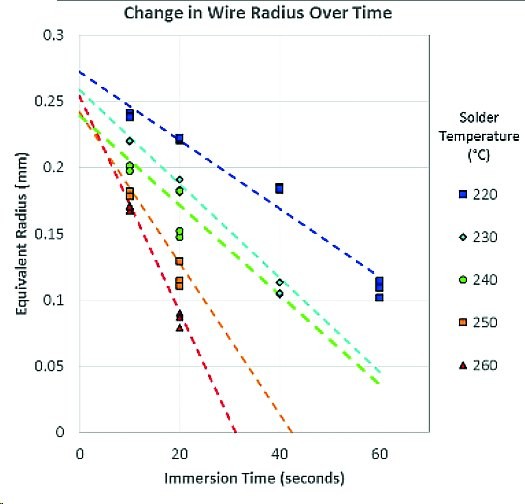 Fig. 3: Reduction of the radii of a gold wire in SAC305 solder at different temperatures and different immersion times
Fig. 3: Reduction of the radii of a gold wire in SAC305 solder at different temperatures and different immersion times
Leaching was of course recognized early on and was also studied for the various lead solders [4]. The studies for the most commonly used lead-free solder SAC305 are somewhat more recent (if you are lucky: 3.0 Ag, 0.5 Cu the rest Sn - preparing a large melt so that these values are exactly maintained is extremely difficult, as we know from casting technology. During cooling alone, stratification occurs which shifts the local proportions by a considerable amount). From the data obtained, it is possible to read off approximately the information required to estimate whether the thickness of a coating will withstand the soldering process or not. However, it must be borne in mind that the quality of a copper layer, for example, depends heavily on the method used to apply it.
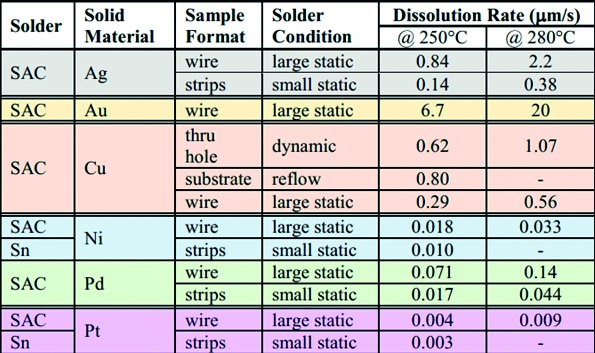 Fig. 4: Dissolution rate of various metals under different conditions and at two temperatures in SAC305
Fig. 4: Dissolution rate of various metals under different conditions and at two temperatures in SAC305
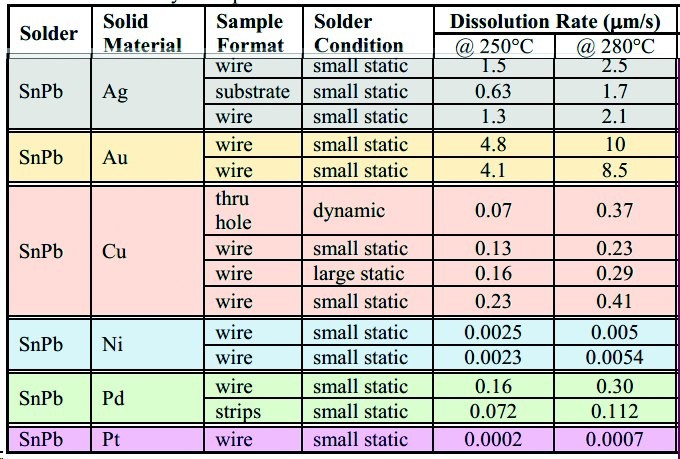 Fig. 5: Similar data for SnPb for comparison
Fig. 5: Similar data for SnPb for comparison
It is also necessary to take a closer look at the studies, as many of them lack calculations, such as consideration of the surface exposed to the solder, as a round wire behaves differently to a flat metallization on the PCB. In addition, the temperature readings on the screen are of course somewhat different from those directly on the object.
It is perhaps noticeable that the deposition of silver wire [in µm/s] accelerates by a factor of 2.6 if the temperature is increased from 250 °C to 280 °C, while the same change for gold results in a value of almost 3. In contrast, nickel, palladium and platinum are attacked quite slowly, but copper surprisingly shows a similar behavior to silver, which should be taken into account when using it especially in reflow processes, where long dwell times above the liquidus of the alloy are common.
Literature and notes:
K. Puttliz; K. Stalter: Handbook of Lead-free Solder Technology for Microelectronics Assemblies, Marcel Decker Inc.
R. Wilcoxon et al.: Solder Joint Integrity Impact of Immersion Silver Surface Finish Thickness for Tin/Lead and Lead-free Solder Processes, SMTA Int. Conf. on Soldering and Reliability (ICSR), May 2008
D. Hillman et al: Dissolution Rate of Specific Elements in SAC305 Solder, Proceedings of SMTA International, Oct. 2018, Rosemont, IL, USA
References:
[1] Quote from Alexander Issayevich Solzhenitsyn (1918-2008), Russian writer and playwright, winner of the Nobel Prize for Literature (main work: The Gulag Archipelago)
[2] Wilhelm Conrad Röntgen (1845-1923), physicist and discoverer of X-rays
[3] J-STD-001 F
[4] W. G. Bader: Dissolution of Au, Ag, Pd, Pt, Cu and NI in a Molten Tin-Lead Solder, Welding Research Supplement, December 1969


