Neuroengineers at Rice University have developed a tiny surgical implant that can electrically stimulate the brain and nervous system without the need for a battery or wired power supply. The neural stimulator draws its power from magnetic energy and is about the size of a grain of rice. It is the first magnetically powered neural stimulator that generates the same type of radio frequency signals as clinically approved, battery-powered implants used to treat epilepsy, Parkinson's disease, chronic pain and other conditions.
The main component of the implant is a thin film of magnetoelectric material that converts magnetic energy directly into an electrical voltage. The method avoids the drawbacks of radio waves, ultrasound, light and even magnetic coils, which have previously been proposed to power tiny wireless implants and have been shown to suffer from interference with living tissue or generate harmful amounts of heat. 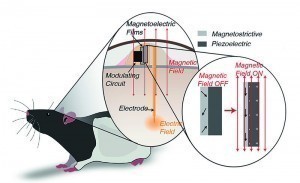 Fig. 2: To demonstrate the viability of magnetoelectrically powered miniature nerve stimulation technology, Rice University neuroengineers developed tiny devices that were placed under the skin of rodents that could move freely in their enclosures. The rodents preferred to stay in parts of the enclosures where a magnetic field activated the stimulator and delivered a small voltage to the reward center of their brains
Fig. 2: To demonstrate the viability of magnetoelectrically powered miniature nerve stimulation technology, Rice University neuroengineers developed tiny devices that were placed under the skin of rodents that could move freely in their enclosures. The rodents preferred to stay in parts of the enclosures where a magnetic field activated the stimulator and delivered a small voltage to the reward center of their brains
To demonstrate the viability of the magnetoelectric technology, the researchers showed that the implants worked in rodents that were fully awake and free to roam in their enclosures. Performing this proof-of-principle demonstration is a huge technological leap from a bench demonstration to something that can actually be used to treat humans. These new materials are ideally suited for wireless bioelectronics and allow miniaturization of electronic implants. Tiny implants that are able to modulate the activity of the brain and nervous system could have far-reaching effects. While battery-powered implants are often used to treat epilepsy and reduce tremors in Parkinson's patients, research has shown that nerve stimulation could be useful in treating depression, obsessive-compulsive disorder and in more than a third of people suffering from chronic, intractable pain, which often leads to anxiety, depression and opioid addiction. The small size of the implants is important because the key to making nerve stimulation therapy more widely available is to create battery-free, wireless devices that are small enough to be implanted without major surgery. Devices the size of a grain of rice could be implanted almost anywhere in the body using a minimally invasive procedure similar to that used to place stents in blocked arteries.
For the animal experiments, the devices were implanted under the skin of rodents so that they could move freely in their enclosures. The stimulator transmitted a small voltage to the reward center of the animals' brains. The rodents preferred to stay in parts of the enclosures where a magnetic field activated the stimulator.
The wireless power supply in the implant was achieved by joining layers of two very different materials into a single film. The first layer, a magnetostrictive film of iron, boron, silicon and carbon, vibrates at the molecular level when placed in a magnetic field. The second, a piezoelectric crystal, converts mechanical voltage directly into electrical voltage. The magnetic field generates voltage in the magnetostrictive material. This generates acoustic waves. If the chip is operated in acoustic resonance mode, the vibrations generate voltage in the piezoelectric half of the film.
While the magnetoelectric films can convert a lot of magnetic energy into electrical energy, they operate at a frequency that is too high to affect the brain cells. The challenge was to modulate the high-frequency signal at a low frequency to which the cells could respond. The modulated biphasic signal must therefore be able to stimulate the neurons without damaging them.


