Question: We are looking for a recipe for electropolishing copper and brass. Old reference books only contain recipes based on chromic acid, which we would like to avoid. We would be grateful for any practical tips.
Answer: Brass and copper can be electrolytically polished in electrolytes of phosphoric acid and alcohols. Aqueous mixtures of 50 % by volume H3PO4 and 30 % by volume of various alcohols are suitable for electropolishing. The achievable gloss and roughness also depend on the alcohol additives selected. Additions of 1-propanol and 2-propanol (isoprapanol), for example, enable the creation of high-gloss surfaces.
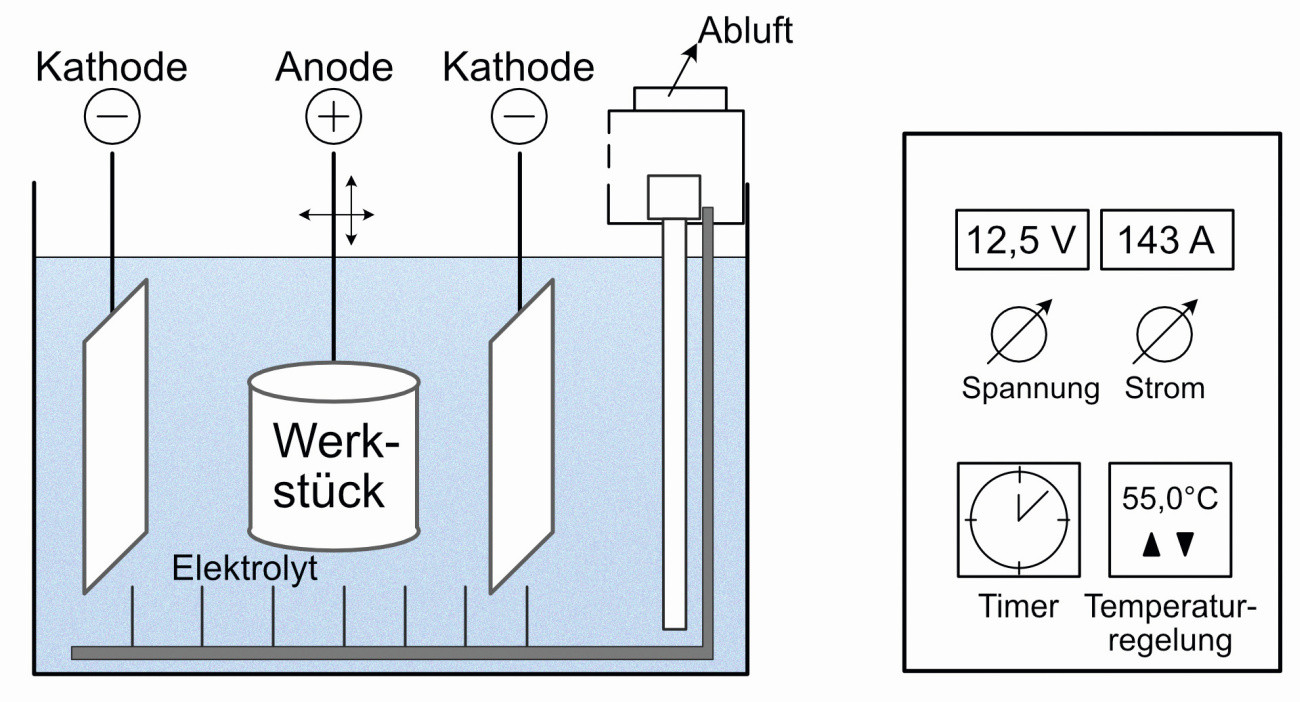 Schematic representation of the electroplating process
Schematic representation of the electroplating process
More recent electrolyte developments are aimed at finding solutions that contain less water and surface-active substances. The basic component of these mixtures is also phosphoric acid. The electrolytes suitable for brass are generally also suitable for the electrolytic polishing, smoothing and deburring of copper. An electrolyte consisting of 55% by weight phosphoric acid, 5% by weight starch, ethylene glycol and methanol is also used for electropolishing copper.
In these electrolytes, copper dissolves in a bivalent solution. The reaction can and will generally take place in several steps. If brass is removed, zinc also goes into solution as a divalent.
Cu -> Cu2+ + 2 e-
Zn->Zn2+ + 2e-
Unwanted side effects can occur during electropolishing. Gas paths are created on the workpiece.
 Aqueous mixtures of various alcohols, as shown here at OTH Oberflächentechnik Hagen, are suitable for electropolishingWaviness ofthe surface is caused by the interplay of rising oxygen bubbles and sinking electrolyte loaded with metal ions. They are all the more pronounced the longer the rising gas bubbles sweep directly along the surface. The electrochemically induced convection leads to locally increased erosion, which is imprinted on the surface in vertical textures, technically known as gas paths. This can be counteracted in various ways.
Aqueous mixtures of various alcohols, as shown here at OTH Oberflächentechnik Hagen, are suitable for electropolishingWaviness ofthe surface is caused by the interplay of rising oxygen bubbles and sinking electrolyte loaded with metal ions. They are all the more pronounced the longer the rising gas bubbles sweep directly along the surface. The electrochemically induced convection leads to locally increased erosion, which is imprinted on the surface in vertical textures, technically known as gas paths. This can be counteracted in various ways.
It is common to move the workpiece or to introduce a further convection flow by blowing in gas. The former measure in particular can influence the formation of gas paths so that these structures are no longer visible.
It is known that electropolishing influences the surface structure down to the nanometer range. There are indications that the underlying processes can be suppressed by using pulsed currents. This makes it possible to reduce the roughness. The resulting higher surface gloss proves that leveling and smoothing occurs in the range of length scales below the wavelength of visible light (400 nm). If suitable parameters are selected, smoother or shinier surfaces can be produced. The position of the workpiece also has an influence on the structure of the surface that is formed during electropolishing. During electropolishing, clearly recognizable removal paths (gas paths) are formed with certain parameter constellations. The positioning of the sample or the metallic workpiece in the gravitational field plays a role in the surface structure that is formed.
In extremely acidic, aqueous electrolytes, oxygen is formed from the electrolyte above a certain voltage, the level of which varies for each electrochemical cell. The fact that gas bubbles repeatedly rise in the same paths at the anode means that more material can be removed locally because the local convection causes an increased exchange of substances. As the removal time increases, these paths "dig in" deeper and deeper until a state is reached from which the type and position of the structure on the workpiece no longer change, only the depth continues to increase. The result is a wavy surface.
Literature
M. Buhlert: Electropolishing, Eugen G. Leuze Verlag, Bad Saulgau, ISBN 978-3-87480-298-7


