Question: We are planning to set up a small laboratory for our electroplating shop and need water of the highest quality. To ensure this, we would like to purchase our own water treatment plant. What modern and cost-effective technologies are available to produce the purest possible water?
Answer: The desire for a self-sufficient pure water supply for a laboratory is widespread. There are various modern and cost-effective technologies available for this purpose, which are described in more detail in the online course "Environmental Technology Part 2 - Energy and Recycling Technology" [1].
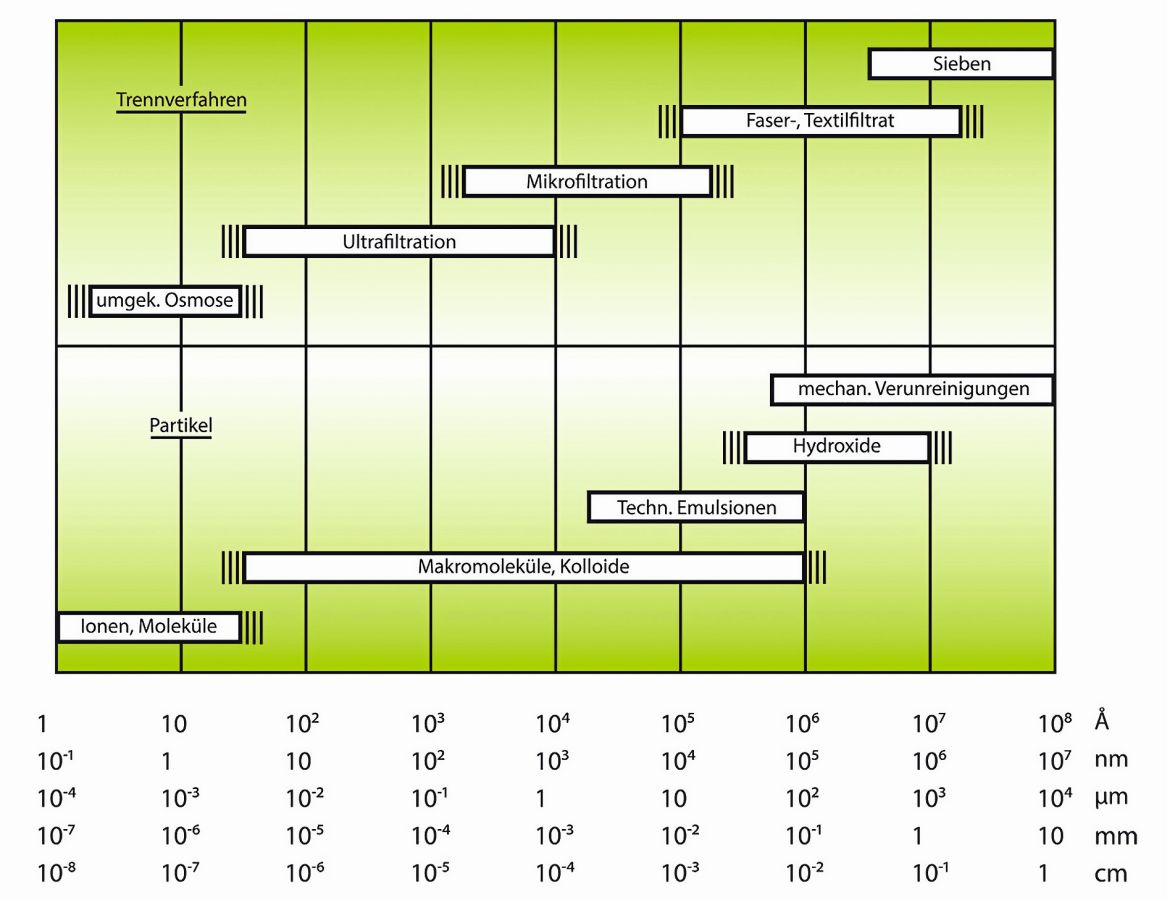
The choice of the right technology depends on the specific purity requirements, the throughput and the available resources. Some technologies, such as ultrafiltration or distillation, are ruled out due to insufficient water quality, cost and time.
Reverse osmosis (RO)
In reverse osmosis, water is forced through a semi-permeable membrane that only allows water molecules to pass through and retains most impurities. The process begins with pre-pressurization, where water is forced through the membrane at high pressure. This membrane filters out dissolved salts, organic substances, bacteria and other impurities. The water that passes through the membrane is called permeate and is very pure, while the remaining water with the impurities is discharged as concentrate or waste water. The wastewater that is produced during reverse osmosis and contains the retained impurities is referred to as concentrate or retentate.
Reverse osmosis is often used when very pure water is required. This is particularly the case in laboratories, in the manufacture of pharmaceutical products, in the food and beverage industry, in electronics production and in seawater desalination. It is also used in private households to produce high quality drinking water.
The cost-effectiveness of reverse osmosis depends heavily on water consumption and the specific requirements for water purity. For small to medium water consumption, such as in households or small laboratories, an RO system can be very useful and economical. For larger industrial applications, the operating costs are higher, but often still justified by the quality of the water produced and the specific purity requirements.
The initial cost of a reverse osmosis system varies greatly depending on size and capacity. Small systems for domestic use can be purchased from around 200 to 500 euros. Medium-sized systems for laboratories or smaller industrial applications range from 1,000 to 10,000 euros. Large industrial systems can cost tens of thousands of euros, depending on their complexity and capacity. In addition to the purchase costs, the operating costs incurred by the regular replacement of membranes, the energy for the operating pressure and the disposal of the wastewater must also be taken into account.
Ion exchanger
In ion exchange technology, unwanted ions in the water are replaced by resin-based materials that remove specific ions from the water and replace them with other, unproblematic ions.
The process begins by passing the water to be treated through a column of ion exchange resins. These resins consist of small, porous plastic spheres that contain either positively charged cations or negatively charged anions. There are two main types of ion exchangers: cation exchangers and anion exchangers. Cation exchange resins exchange positively charged ions such as calcium and magnesium for hydrogen ions, while anion exchange resins exchange negatively charged ions such as chloride and sulphate for hydroxide ions.
As the water flows through the resin column, the unwanted ions bind to the resin and are replaced by the corresponding exchange ions. The resulting water leaving the column is free of the unwanted ions and has a higher purity. We assume that such systems are already in use in your production.
The exchange process is continuous until the capacity of the resin is exhausted. The resin must then be regenerated, which is done by rinsing with a concentrated solution of the exchange ions (e.g. salt solution in water softening systems, HCl and NaOH in industrial systems).
The purchase costs for ion exchange systems vary greatly depending on size and application. Small systems for households can cost a few hundred euros, while larger industrial systems can cost tens of thousands of euros. Operating costs arise from the regular need for regenerants and the replacement or preparation of the resins as well as costs for the waste water due to the so-called eluates. In particularly small laboratories, replaceable cartridges are used, which are offered by suppliers. The advantage is that you do not have to worry about regeneration.
Exchangeable cartridges can be so-called mixed-bed exchangers. Mixed bed exchangers contain both cation exchanger and anion exchanger resins in a single container or cartridge. They can bring water to a very high purity level, often comparable to the quality achieved by multi-stage processes such as reverse osmosis and separate ion exchangers. They are easy to use and maintain as they do not require separate regeneration steps.
 Filtration process
Filtration process
Electrodeionization (EDI)
Electrodeionization is an advanced water treatment technology that combines ion exchange resins and electric fields to continuously produce high purity water. It is often used as a post-treatment step after reverse osmosis to further increase the purity of the water and reduce conductivity to very low levels.
The EDI process begins with the supply of pre-purified water. The water flows through an EDI cell consisting of several thin layers, including ion exchange resins in the form of a mixed bed exchanger and ion exchange membranes. These layers are arranged in such a way that they alternately allow cations and anions to pass through.
An electric field is applied in the EDI cell, which forces the ions in the water to move through the ion exchange membranes. The ion exchange resins serve as conductive media that absorb ions and transport them through the membranes.
A major advantage of EDI is that the ion exchange resins are continuously regenerated. The electric field ensures that the resins lose their ionic charge and are immediately available for ion exchange again. This eliminates the need for chemical regeneration agents such as acids or alkalis, which are required in conventional ion exchange systems.
EDI is used in various applications where extremely pure water is required. These include laboratories, the pharmaceutical industry, the electronics industry and power stations.
The cost-effectiveness of EDI systems depends on the specific application requirements and the size of the system. The initial cost of EDI systems can be higher than conventional ion exchangers, but the lower operating costs and the benefits of continuous, chemical-free regeneration often make them more economical in the long term. Typical EDI systems for industrial applications can cost several thousand to tens of thousands of euros, depending on capacity and specific requirements.
Pre- and post-filtration
Depending on the water quality, further filtration may or may not be necessary, for example to remove organic impurities and bacteria. Activated carbon filters and UV disinfection can be used here.
UV disinfection uses ultraviolet light to kill microorganisms in the water. The water is either passed through a UV tube or a storage tank is created in which UV lamps are installed. Ultrafiltration (UF) could also serve as a "pre-filter". The UF process begins by feeding raw water into a UF system. The water is passed through the membrane under pressure, very similar to reverse osmosis. The membrane acts as a barrier, retaining larger particles, bacteria, viruses and colloids, while smaller molecules and dissolved substances pass through the pores. The pores of UF membranes are typically between 0.01 and 0.1 micrometers in size.
Although both UF and RO are membrane filtration technologies, they differ significantly in terms of pore size, separation performance, required operating conditions and typical applications. With UF alone, you will not consistently achieve the water quality required for a laboratory.
Conclusion
Deciding on the right technology depends primarily on the water quality you require and your budget. Throughput and space requirements may also play a role.
For smaller laboratories, we recommend starting with exchanger cartridges, but keeping the option of reverse osmosis open. If you require particularly pure water, we recommend a combination of reverse osmosis and electrodeionization.
SOURCE
[1] GTFY course "Environmental Technology Part 2 - Energy and Recycling Technology " https://www.galvanotechnik-for-you.de/uebersicht-kurse/umwelttechnik-teil-2-energie-und-recyclingtechnik/







