Using plasma-chemical oxidation - a process between plasma physics and electroplating - a coating of a magnesium alloy implant that is harmless to the body has been developed.
Biodegradable materials have been attracting the attention of scientists and doctors for a few years now for the use of implants in reconstructive medicine. As part of an international research project, the biocompatible coatings were formed on a MgCa1 alloy using plasma chemical oxidation (PCO) and their morphology, structure, phase composition, corrosion and biological properties were investigated. The coated samples showed remarkable anti-corrosion properties compared to an uncoated magnesium alloy, they did not affect the viability of the cells and were non-toxic according to ISO 10993-5: 2009.
Great interest in magnesium alloys
The regenerative approach is becoming increasingly important in medicine, meaning that the demand for orthopaedic implants with bioactive and antibacterial coatings remains high. Implantable systems that are resorbable over time and can be replaced by new bone tissue are of interest for medical technology. For this reason, magnesium alloys have recently gained a lot of attention. The growing market for implants requires advanced biodegradable magnesium alloys with controllable dissolution rates.
Due to their specific strength and stiffness, low density, bone-like mechanical properties [1], biocompatibility and degradation behavior [2-6], magnesium alloys are used in various fields such as orthopedics and the cardiovascular system. Despite all these positive properties, magnesium alloys still have too high a corrosion rate, so that degradation starts before the end of the healing process, the antibacterial properties are not yet sufficiently developed and hydrogen is released during the corrosion/degradation process. This leads to an increase in the pH value in the surrounding tissue and can trigger apoptosis and necrosis of tissue cells [7, 8].
Over the years, various ways of improving the performance of magnesium alloys have been proposed:
- by alloying with corrosion-inhibiting elements
- by mechanical pretreatment
- by surface treatment [5, 9, 10].
The first method is chronologically the oldest method. Various elements such as Al, Zn, Mn, Si, Sr, Zr, Cu and rare earths are added to the magnesium alloy in order to improve various mechanical, physical and chemical properties of the alloy [3, 4, 6, 9-14]. Calcium is also used in magnesium alloys to refine the microstructure and increase the thermal stability of the β-phase, resulting in improved yield strength and creep resistance [15-17].
Surface treatment of magnesium alloys is a promising way to influence or even control the corrosion rate. Numerous surface treatment options for magnesium alloys have been investigated, but the most commonly used method is the coating of magnesium alloys using the plasma chemical oxidation (PCO) process, also known as micro arc oxidation (MAO) or plasma electrolytic oxidation (PEO). Surmenev has published a review in which all methods for the production of CaP coatings are well described with their advantages and disadvantages [18].
Plasma chemical oxidation
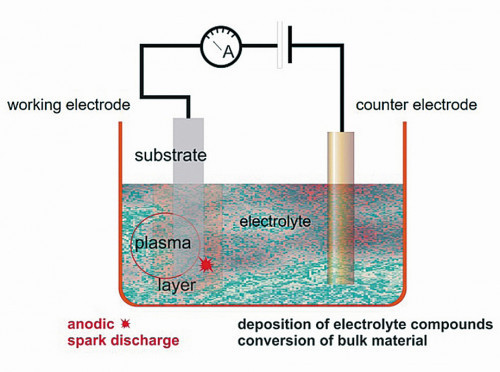
Plasma chemical oxidation (PCO) was also selected for coating production in this international research project. The PCO process can be classified between plasma physics and electroplating and generates surface discharges at the anode and the formation of a dielectric in an aqueous electrolyte. The main features of this discharge are the formation of oxide from the substrate material and the incorporation of additional electrolyte components into the layer. This makes it possible to vary the layer properties within wide limits both through the electrical parameters and the chemical composition of the electrolyte. With no other anodic oxidation process is it possible to influence the layer properties in such a way through the composition of the electrolyte and to specifically incorporate desired elements/compounds into the layer formed. One of the aims of the research project was to produce CaP coatings for the magnesium alloy MgCa1, which should also have a zinc content. The element zinc was chosen because it has high antibacterial properties against various types of bacteria or fungi [9, 14, 19-21]. These doped layers have already been comprehensively characterized in other publications of the research project [22], so that only a brief overview of the main results will be given here.
The MgCa1 alloy used for these investigations was produced at Helmholz Zentrum Hereon GmbH in Geesthacht as ingots with a diameter of 60 mm and a length of 170 mm. The ingots were then further processed by extrusion to obtain rods with a diameter of 15 mm. By mechanical processing (CNC turning), the rods were divided into 3 mm thick samples, so that the sample dimensions of Ø 15 x 3 mm were obtained, which were advantageous for the corresponding biological tests and investigations (Fig. 1).
|
Electrolyte name |
Main components |
|
CaP |
*Potassium dihydrogen phosphate [KH2PO4] *Ammonium dihydrogen phosphate [NH4H2PO4] *Sodium - Calcium - EDTA [C10H12N2O8CaNa2] |
|
CaP+Zn |
*Potassium dihydrogen phosphate [KH2PO4] *Ammonium dihydrogen phosphate [NH4H2PO4] *Sodium - calcium - EDTA [C10H12N2O8CaNa2] *Zinc nitrate [Zn(NO3)2] |
The PCO process and specially synthesized electrolytes were used to functionalize the samples from the MgCa1 alloy. The schematic representation of the process can be seen in Figure 2. The electrolytes listed in Table 1 with their respective function-determining main components were used for the coating. All coatings were carried out using a pulsed bipolar current generator with a pulse duration of 1 ms. The ratio of anodic to cathodic charge was set to a value of 1. The anodic current density was set at 5 A/dm2. The parameter for ending the coating process was defined as the bath voltage at which a desired coating thickness of 10 µm was achieved. Regardless of the coating time, the process was terminated when the set current had dropped to 50 % of the initial current. This two-part mode of operation, in which the first phase of the PCO process is characterized by galvanostatic polarization and the second phase by potentiostatic polarization, has had a positive effect on the properties of the PCO coatings in all investigations carried out to date.
Characterization
The electrochemical tests were carried out using a potentiostat / galvanostat VersaSTAT 3 (Princeton Applied Research, USA) and the data were recorded using the VersaStudio software in accordance with the ASTM G5-94 standard. The electrolyte used was an SBF solution (pH=7.2) at 37±0.5 °C. As a result of these electrochemical corrosion tests, the most important electrochemical parameters were extracted using table curves and presented in Table 2. The evaluation of these parameters shows that the two coated variants exhibit better corrosion resistance than the uncoated MgCa1 substrate, with the MgCa1_CaP+Zn variant having the lowest corrosion current density value. This was followed by the MgCa1_CaP variant, which was very similar to the uncoated MgCa1 alloy. For the polarization resistance (Rp), the highest value was found for the Zn-doped coating, indicating that this doping of the CaP coating significantly improves the corrosion resistance. Considering the value of the corrosion current density of the coated samples and the uncoated MgCa1 substrate, the protective effect (Pe) against SBF attacks was calculated based on the procedure described in Refs. [12, 23-25]. From the comparison of the results, it is clear that Zn doping can provide a high level of protection against SBF attacks in the human body. The cytotoxicity of the samples was determined by indirect tests with sample extracts; this is described in [22].
|
Sample designation |
Ecorr (V) |
icorr (µA cm-2) |
Rp (Ωcm2) |
|
MgCa1 (substrate) |
-1.558 |
994.519 |
56.9165 |
|
MgCa1_CaP |
-1.554 |
376.842 |
92.8275 |
|
MgCa1_CaP+Zn |
-1.372 |
78.348 |
1952.110 |
The results
 Fig. 3: Typical spark dischargeThePCO coating process resulted in a rougher, more porous and more hydrophilic surface for both coating variants compared to the uncoated substrate material. This favored the adhesion and growth of the investigated cells. All samples proved to be cytocompatible. The in vitro cultivation of the samples with Saos-2 cells led to some changes in the elemental composition of the alloys due to their biodegradation. The Zn-doped coatings contained between 0.94 and 1.16 atomic % Zn.
Fig. 3: Typical spark dischargeThePCO coating process resulted in a rougher, more porous and more hydrophilic surface for both coating variants compared to the uncoated substrate material. This favored the adhesion and growth of the investigated cells. All samples proved to be cytocompatible. The in vitro cultivation of the samples with Saos-2 cells led to some changes in the elemental composition of the alloys due to their biodegradation. The Zn-doped coatings contained between 0.94 and 1.16 atomic % Zn.
The number of cells adhering to the surface of Zn-doped coatings (2.6 × 104 cells/cm2) was higher than the number of cells adhering to the surface of the undoped coating (1.0 × 103 cells/cm2). The adhesion of the CaP coating to the MgCa1 alloy substrate was improved by the addition of Zn. The doping with Zn also influenced the microstructure of the CaP coating, which in turn affected the mechanical properties. The hardness of the coating was 4.58 GPa for the MgCa1_CaP variant and 1.62 GPa for the MgCa1_CaP+Zn variant, while the values measured for the modulus of elasticity were 81.90 GPa for the MgCa1_CaP variant and 60 GPa for the MgCa1_CaP+Zn variant. As a result, the coating produces a material that resists plastic deformation during loading. The coating adhesion to the substrate was improved by the Zn doping.
This variant showed only a minimal change in the coating under in-vitro conditions, which promises a sustainable corrosion protection effect for long-term use under physiological conditions. In summary, the CaP-coated and Zn-doped magnesium-calcium alloys developed in this research project may be novel and promising candidates for biodegradable metallic bone tissue implants in terms of corrosion resistance and osteoconductivity.
Summary
The experiments carried out in this research project and the resulting findings allow the following conclusions to be drawn:
- CaP-based coatings can be produced using the PCO process. The properties required for degradable implants can be positively influenced by Zn doping.
- In the electrochemical tests, the best behavior was found in the following order:
- MgCa1_CaP+Zn > MgCa1_CaP > MgCa1
- The coating with a Zn doping showed the best corrosion protection in SBF solution at 37 °C
- The Zn-doped coatings are characterized by a stronger negatively charged surface than the undoped coatings of the samples. The electrical charge of the Zn-doped coatings is the most stable compared to the undoped coatings.
Literature
[1] A. Abdal-Hay; M. Dewidar; J.K. Lim: Biocorrosion behavior and cell viability of adhesive polymer coated magnesium based alloys for medical implants, Appl. Surf. Sci. 261 (2012) 536-546, https://doi.org/10.1016/j.apsusc.2012.08.051
[2] Witte: The history of biodegradable magnesium implants: A review, Acta Biomater. 6 (2010) 1680-1692, https://doi.org/10.1016/j.actbio.2010.02.028
[3] H.S. Brar; M.O. Platt; M. Sarntinoranont; P.I. Martin; M.V. Manuel: Magnesium as a biodegradable and bioabsorbable material for medical implants, JOM. 61 (2009) 31-34, https://doi.org/10.1007/s11837-009-0129-0
[4] Y. Zheng: Magnesium Alloys as Degradable Biomaterials, 2015, https://doi.org/10.1201/b18932
[5] W.H. Sillekens; D. Bormann: Biomedical applications of magnesium alloys, in: Adv. Wrought Magnes. Alloy., 2012, https://doi.org/10.1533/9780857093844.3.427
[6] P.K. Bowen; W.H. Sillekens: Magnesium-based biodegradable implants, Jom. 68 (2016) 1175-1176, https://doi.org/https://doi.org/10.1007/s11837-016-1864-7
[7] Z. Yao; L. Li; Z. Jiang: Adjustment of the ratio of Ca/P in the ceramic coating on Mg alloy by plasma electrolytic oxidation, Appl. Surf. Sci. 255 (2009) 6724-6728, https://doi.org/10.1016/j.apsusc.2009.02.082
[8] O.I. Velikokhatnyi; P.N. Kumta: First-principles studies on alloying and simplified thermodynamic aqueous chemical stability of calcium-, zinc-, aluminum-, yttrium- and iron-doped magnesium alloys, Acta Biomater. 6 (2010) 1698-1704, https://doi.org/10.1016/j.actbio.2009.08.016
[9] J. Gonzalez; R.Q. Hou; E.P.S. Nidadavolu; R. Willumeit-Römer; F. Feyerabend: Magnesium degradation under physiological conditions - Best practice, Bioact. Mater. 3 (2018) 174-185, https://doi.org/10.1016/j.bioactmat.2018.01.003
[10] G. Amirhossein; R.A.K. Mohammed; R.A. Mohamed: Biodegradable Metals (Biodegradable Magnesium Alloys), in: Trauma Plat. Syst. Biomech. Mater. Biol. Clin. Asp., Elsevier, 2017: 143-158, https://doi.org/https://doi.org/10.1016/B978-0-12-804634-0.00008-2
[11] Nouha Loukil: Alloying Elements of Magnesium Alloys: A Literature Review, in: T.A. Tański, P. Jarka (Eds.), Magnes. Alloy, IntechOpen, 2021, https://doi.org/10.5772/intechopen.96232
[12] D.M. Vranceanu; I.C. Ionescu; E. Ungureanu; M.O. Cojocaru; A. Vladescu; C.M. Cotrut: Magnesium doped hydroxyapatite-based coatings obtained by pulsed galvanostatic electrochemical deposition with adjustable electrochemical behavior, Coatings. 10 (2020) 727-731, https://doi.org/10.3390/COATINGS10080727
[13] A.H.M. Sanchez; B.J.C. Luthringer; F. Feyerabend; R. Willumei: Mg and Mg alloys: How comparable are in vitro and in vivo corrosion rates? A review, Acta Biomater. 13 (2015) 16-31, https://doi.org/10.1016/j.actbio.2014.11.048
[14] J.F. Hernández-Sierra; F. Ruiz; D.C. Cruz Pena; F. Martínez-Gutiérrez; A.E. Martínez; A. de Jesús Pozos Guillén; H. Tapia-Pérez; G. Martínez Castañón: The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold, Nanomedicine Nanotechnology, Biol. Med. 4 (2008) 237-240, https://doi.org/10.1016/j.nano.2008.04.005
[15] U. Riaz; I. Shabib; W. Haider: The current trends of Mg alloys in biomedical applications - A review, J. Biomed. Mater. Res. - Part B Appl. Biomater. (2019), https://doi.org/10.1002/jbm.b.34290
[16] D. Wenwen; S. Yangshan; M. Xuegang; X. Feng; Z. Min; W. Dengyun: Microstructure and mechanical properties of Mg-Al based alloy with calcium and rare earth additions, Mater. Sci. Eng. A. 356 (2003) 1-7, https://doi.org/10.1016/S0921-5093(02)00551-8
[17] Y. Wan; G. Xiong; H. Luo; F. He; Y. Huang; X. Zhou: Preparation and characterization of a new biomedical magnesium-calcium alloy, Mater. Des. 29 (2008) 2034-2037, https://doi.org/10.1016/j.matdes.2008.04.017
[18] R.A. Surmenev: A review of plasma-assisted methods for calcium phosphate-based coatings fabrication, Surf. Coatings Technol. 206 (2012) 2035-2056, https://doi.org/10.1016/j.surfcoat.2011.11.002
[19] M.M. Almoudi; A.S. Hussein; M.I. Abu Hassan; N. Mohamad Zain: A systematic review on antibacterial activity of zinc against Streptococcus mutans, Saudi Dent. J. 30 (2018) 283-291, https://doi.org/10.1016/j.sdentj.2018.06.003
[20] D. Predoi; S.L. Iconaru; M.V. Predoi; M. Motelica-Heino; R. Guegan; N. Buton: Evaluation of antibacterial activity of zinc-doped hydroxyapatite colloids and dispersion stability using ultrasounds, Nanomaterials. 9 (2019) 515-521, https://doi.org/10.3390/nano9040515
[21] N. Ohtsu; Y. Kakuchi; T. Ohtsuki: Antibacterial effect of zinc oxide/hydroxyapatite coatings prepared by chemical solution deposition, Appl. Surf. Sci. 445 (2018) 596-600, https://doi.org/10.1016/j.apsusc.2017.09.101
[22] D.G. Tamay; S. Gokyer; J. Schmidt; A. Vladescu; P. Yilgor Huri; V. Hasirci; N. Hasirci: Corrosion Resistance and Cytocompatibility of Magnesium-Calcium Alloys Modified with Zinc- or Gallium-Doped Calcium Phosphate Coatings, ACS Appl. Mater. Interfaces. 14 (2022) 104-122, https://doi.org/10.1021/acsami.1c16307
[23] M.P. Seah; W.A. Dench: Quantitative electron spectroscopy of surfaces: a standard data base for electron inelastic mean free paths in solids, Surf. Interface Anal. 1 (1979) 2-11
[24] R. Akmene; A. Balodis; Y. Dekhtyar; G. Markelova; Y. Matvejevs; L. Rozenfeld; S. Sagalovich; Y. Smirnov; A. Tolkachov; A. Upminsh: Exoelectron emission spectrometre complete set of surface local investigation, Surfaces, Phys Chem Mech Surf. 8 (1993) 125-128
[25] ISO 16773-2: Electrochemical impedance spectroscopy (EIS) on coated and uncoated metallic specimens - Part 2: Collection of datap, 2016