Researchers at ETH Zurich have developed a method with which drugs can be released in the brain with pinpoint accuracy. This could make it possible in future to deliver psychotropic drugs, chemotherapeutic agents and other medicines only to those regions of the brain where this is desired for medical reasons.
Today, this is practically impossible - drugs reach the whole brain and the whole body via the blood, which in some cases is the cause of side effects. The new method is non-invasive - the precise drug release in the brain is controlled from outside the head using ultrasound. The scientists, led by Mehmet Fatih Yanik, Professor of Neurotechnology, report this in the journal Nature Communications. In order to prevent a drug from exerting its activity throughout the entire body and brain, the new method uses special carriers that wrap the drug in spherical lipid vesicles attached to gas-containing, ultrasound-sensitive microbubbles. These are injected into the blood and thus reach the brain.
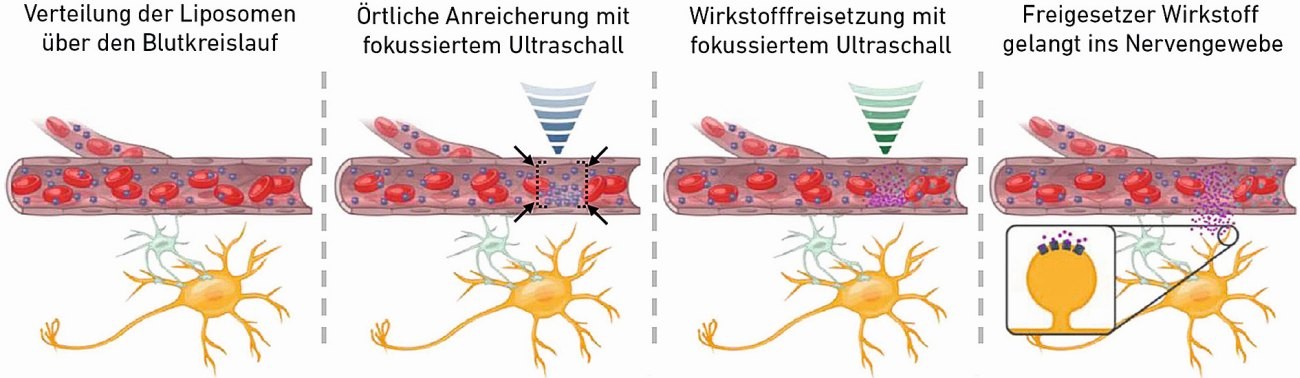 Fig. 1: Schematic of the two-stage process for the accumulation and finally local release of encapsulated active ingredients (Image: Ozdas et al. Nature Communications 2020)
Fig. 1: Schematic of the two-stage process for the accumulation and finally local release of encapsulated active ingredients (Image: Ozdas et al. Nature Communications 2020)
The scientists then use focused ultrasound in a two-stage process. Focused ultrasound is already used in cancer medicine today to destroy cancerous tissue at precisely defined points in the tissue. In the new application, however, the scientists work with much lower energy, which does not damage the tissue.
Enriching drugs with sound
In a first step, the scientists use low-energy ultrasound waves to enrich the drug carriers at the desired location in the brain. "You can imagine that we use ultrasound pulses to create a kind of virtual sound wave cage at the desired location. Driven by the blood circulation, the drug carriers are flushed through the entire brain. However, those that get into the cage can no longer find their way out," explains ETH Professor Yanik.
In a second step, the researchers cause the drug carriers to vibrate at this location using higher ultrasound energy. Frictional forces destroy the outer membrane of the containers, the active ingredient is released and absorbed by the nerve tissue at this point.
The researchers have demonstrated the effectiveness of the new method in experiments with rats. They encapsulated a neuroinhibitor in the drug carriers. This enabled them to block a specific neuronal network that connects two areas of the brain. In the experiments, the scientists were able to show that only this specific part of the network was blocked and that the drug did not affect the entire brain.
More efficient drug administration
"Because our method allows us to accumulate drugs in the body where their effect is desired, a much lower dose is sufficient," says Yanik. For their experiment in rats, for example, they needed 1300 times less active ingredient than would normally be necessary.
Other scientists have previously tried to use focused ultrasound to improve the delivery of drugs to certain regions of the brain. In those approaches, however, the active ingredients were not enriched locally, but the blood vessels were damaged locally in order to increase the transport of active ingredients from the blood into the nerve tissue. However, this approach can have harmful long-term consequences. "In our approach, however, the physiological barrier of blood circulation and nerve tissue remains intact," says Yanik.
The scientists are currently testing the effectiveness of their method in animal models of mental illnesses and neurological disorders, for example to treat anxiety disorders and to treat brain tumors in surgically inaccessible areas. Only when the effectiveness and benefits of the method are confirmed in animals will the researchers be able to advance the use of the method in humans.
Ozdas MS, Shah AS, Johnson PM, Patel N, Marks M, Yasar TB, Stalder U, Bigler L, von der Behrens W, Sirsi SR, Yanik MF: Non-invasive molecularly-specific millimeterresolution manipulation of brain circuits by ultrasound-mediated aggregation and uncaging of drug carriers. Nature Communications, October 1, 2020, doi: 10.1038/s41467-020-18059-7
Source: ETH Zurich, Fabio Bergamin; This research project has received funding from the European Union's Horizon 2020 research and innovation program.


