Nano-particles are also taking on more and more important tasks in medicine. They have recently been used instead of antibiotics.
Filters against viruses [1]
Viruses can spread in different ways. Some do this via droplets and aerosols, such as the new coronavirus, while others can be found in water, such as rota or enteroviruses. Until now, such aquatic viruses have been removed from water using nanofiltration or the reverse osmosis process, which is expensive and pollutes the environment. Nanofilters, for example, consist of petroleum-based raw materials, while reverse osmosis requires a relatively large amount of energy.
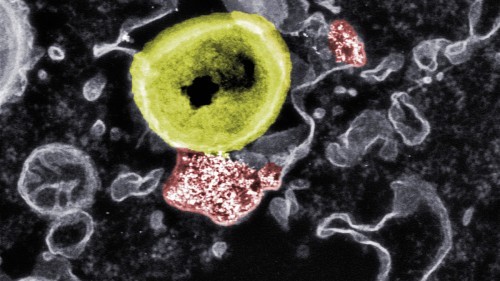 Fig. 2: Deadly contact: Researchers at Empa and ETH have developed nanoparticles (red) that kill resistant bacteria (yellow)Nowan international team of researchers led by Raffaele Mezzenga, Professor of Food and Soft Materials at ETH Zurich, has developed a new filter membrane that is highly effective at eliminating viruses from water and is environmentally friendly. The researchers used natural starting materials for its production.
Fig. 2: Deadly contact: Researchers at Empa and ETH have developed nanoparticles (red) that kill resistant bacteria (yellow)Nowan international team of researchers led by Raffaele Mezzenga, Professor of Food and Soft Materials at ETH Zurich, has developed a new filter membrane that is highly effective at eliminating viruses from water and is environmentally friendly. The researchers used natural starting materials for its production.
The filter membrane is based on the same principle that Mezzenga and his colleagues developed to remove heavy or precious metals from water. The membrane is based on denatured whey proteins, which assemble into tiny fibers known as amyloid fibrils. The researchers have now combined this fibril framework with nanoparticles of iron hydroxide (Fe-O-HO).
The membrane is relatively easy to produce. To produce the fibrils, whey proteins from milk processing are placed in acid and heated to 90 °C. This stretches the proteins and causes them to form a membrane. This causes the proteins to stretch and attach to each other, creating fibrils. The nanoparticles can be produced in the same reaction vessel as the fibrils by raising the pH value and adding iron salt. This "decomposes" into iron-hydroxide nanoparticles, which attach to the amyloid fibrils. In this case, Mezzenga and his colleagues used cellulose as a carrier for the membrane. The combination of amyloid fibrils and iron hydroxide nanoparticles makes the membrane a highly effective and efficient trap for various viruses circulating in water. The positively charged iron oxide electrostatically attracts the negatively charged viruses and inactivates them. The amyloid fibrils alone would not be able to do this because, like the virus particles, they are also negatively charged at a neutral pH value. However, the fibrils are the ideal carrier for the iron oxide nanoparticles.
The membrane eliminates various viruses in the water, including envelopeless adenoviruses, retroviruses and enteroviruses, which can cause dangerous gastrointestinal infections. Every year, around half a million people - often small children in developing and emerging countries - die from infections with enteroviruses. These are extremely tough and acid-resistant and remain in the water for a very long time. The filter membrane could therefore help prevent such infections, especially in poorer countries.
The membrane also eliminates pN1 influenza viruses and the Sars-Cov-2 virus from water very efficiently. In the filtered samples, the concentration of both viruses was below the detection limit, which equates to an almost complete elimination of these pathogens.
"We are aware that the new coronavirus is mainly transmitted via droplets and aerosols. But even then, it must always be surrounded by water. The fact that we can also remove it very efficiently from water impressively underlines the broad applicability of our membrane," says Mezzenga.
The membrane is primarily designed for use in sewage treatment plants or in drinking water treatment. However, it could also be used in air filtration systems or even in masks. It consists exclusively of biocompatible materials, so it could simply be composted after use, and can be produced with minimal energy input. It therefore has an excellent environmental footprint, as the researchers also demonstrate in their study. The filtration is passive, i.e. does not require any additional energy, which makes its operationCO2-neutral and predestines it for various locations.
In addition to Raffaele Mezzenga's laboratory, scientists from several Swiss universities were also involved in the work, including virus specialists from the Universities of Zurich, Lausanne and Geneva, EPFL, the University of Cagliari and the ETH spin-off BluAct. The company holds the patent on this new technology.
Fighting dangerous bacteria with nanoparticles [3]
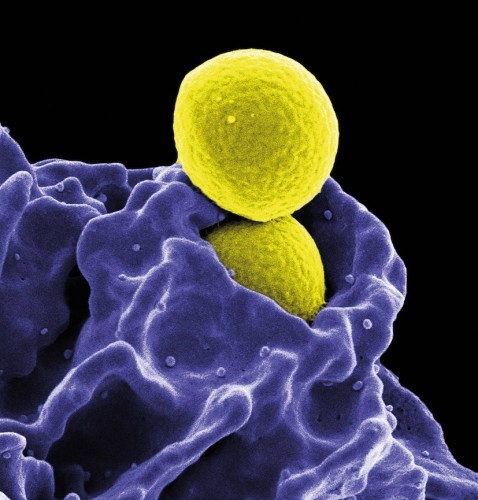 Fig. 3: Neutrophils swallow MRSAIn thearms race "mankind versus bacteria", bacteria are currently in the lead. Our former miracle weapons, antibiotics, are increasingly failing against germs that use tricky maneuvers to protect themselves from the effects of the drugs. Some species even retreat into the interior of human body cells, where they remain unaffected by the immune system. These particularly feared pathogens include so-called multi-resistant staphylococci (MRSA), which can cause life-threatening diseases such as blood poisoning or pneumonia.
Fig. 3: Neutrophils swallow MRSAIn thearms race "mankind versus bacteria", bacteria are currently in the lead. Our former miracle weapons, antibiotics, are increasingly failing against germs that use tricky maneuvers to protect themselves from the effects of the drugs. Some species even retreat into the interior of human body cells, where they remain unaffected by the immune system. These particularly feared pathogens include so-called multi-resistant staphylococci (MRSA), which can cause life-threatening diseases such as blood poisoning or pneumonia.
In order to track down the germs in their hiding places and render them harmless, a team of researchers from Empa and ETH Zurich has now developed nanoparticles that use a completely different mechanism of action than conventional antibiotics: While antibiotics have difficulty penetrating body cells, these nanoparticles are able to infiltrate the inside of the infected cell due to their small size and composition. Once there, they fight the bacteria.
Inge Herrmann and Tino Matter's team used the material cerium oxide for this purpose, which has an antibacterial and anti-inflammatory effect in its nanoparticle form. The researchers combined the nanoparticles with a bioactive ceramic material known as bioglass. Bioglass is interesting for medicine as it has versatile regenerative properties and is used, for example, for the reconstruction of bones and soft tissue.
Finally, nanoparticle hybrids of cerium oxide and bioglass were produced using flame synthesis. The particles have already been successfully used as wound adhesives, whereby several interesting properties can be used simultaneously: Thanks to the nanoparticles, bleeding can be stopped, inflammation dampened and wound healing accelerated. In addition, the novel particles have a significant effect against bacteria, while the treatment is well tolerated by human cells. The new technology was only recently successfully patented. The team has now published its results in the journal "Nanoscale" in the "Emerging Investigator Collection 2021" [4]. Tino Matter is currently working on bringing the new technology to market maturity. His start-up anavo medical has already celebrated several successes - including being one of the three finalists for the Swiss Technology Award.
Among bacteria, there are some particularly tricky pathogens that penetrate body cells and are invisible to the immune system. This allows them to survive times when the body's defenses are on alert. This phenomenon is also known for staphylococci. They can withdraw into cells of the skin, connective tissue, bones and the immune system. The mechanism of this persistence is not yet fully understood.
Staphylococci are mostly harmless germs that can be found on the skin and mucous membranes. Under certain conditions, however, the bacteria flood the body and trigger severe inflammation, including toxic shock or blood poisoning. This makes staphylococci the main cause of death from infections with just one type of pathogen.
The increasing number of staphylococcal infections that no longer respond to treatment with antibiotics is particularly precarious. MRSA, multi-resistant germs, are particularly feared in hospitals, where they are nosocomial pathogens that cause difficult-to-treat wound infections or colonize catheters and equipment. In total, around 75,000 hospital infections occur in Switzerland every year, 12,000 of which are fatal.
The researchers were able to demonstrate the interactions between the hybrid nanoparticles, the body cells and the germs using electron microscopy studies, among other things. When infected cells were treated with the nanoparticles, the bacteria inside the cells began to dissolve. If, on the other hand, the uptake of the hybrid particles was specifically blocked by the researchers, the antibacterial effect also stopped.
The exact mechanism of action of the cerium-containing particles is not yet fully understood. It has been proven that other metals also have antimicrobial effects. However, cerium is less toxic to body cells than silver, for example. The researchers currently assume that the nanoparticles act on the cell membrane of the bacteria, creating reactive oxygen compounds that lead to the destruction of the germs. As the membrane of human cells is structured differently, body cells are spared this process.
The researchers believe that such a mechanism would presumably reduce the development of resistance. "In addition, the cerium oxide particles regenerate again over time, so that the oxidative effect of the nanoparticles on the bacteria starts up again," says Empa researcher Tino Matter. In this way, the cerium particles could achieve a lasting effect.
Next, the researchers want to analyze the interactions of the particles in the infection process in more detail in order to further optimize the structure and composition of the nanoparticles. The aim is to develop a simple, robust antibacterial agent that is effective inside infected cells.
Literature
[1] Source: ETH News Peter Rück
[2] Palika, A.; Armanious, A.; Rahimi, A. et al.: An anti-viral trap made of protein nanofibrils and iron oxyhydroxide nanoparticles, Nature Nanotechnology, 2021, published online June 3, doi: 10.1038/s41565-021-00920-5
[3] Source: EMPA News Andrea Six
[4] Matter, M.T.; Doppegieter, M.; Gogos, A.; Ren, Q.; Keevend, K.; Herrmann, I.K.: Inorganic nanohybrids combat antibiotic-resistant bacteria hiding within human macrophages, Nanoscale (2021), https://doi.org/10.1039/D0NR08285


