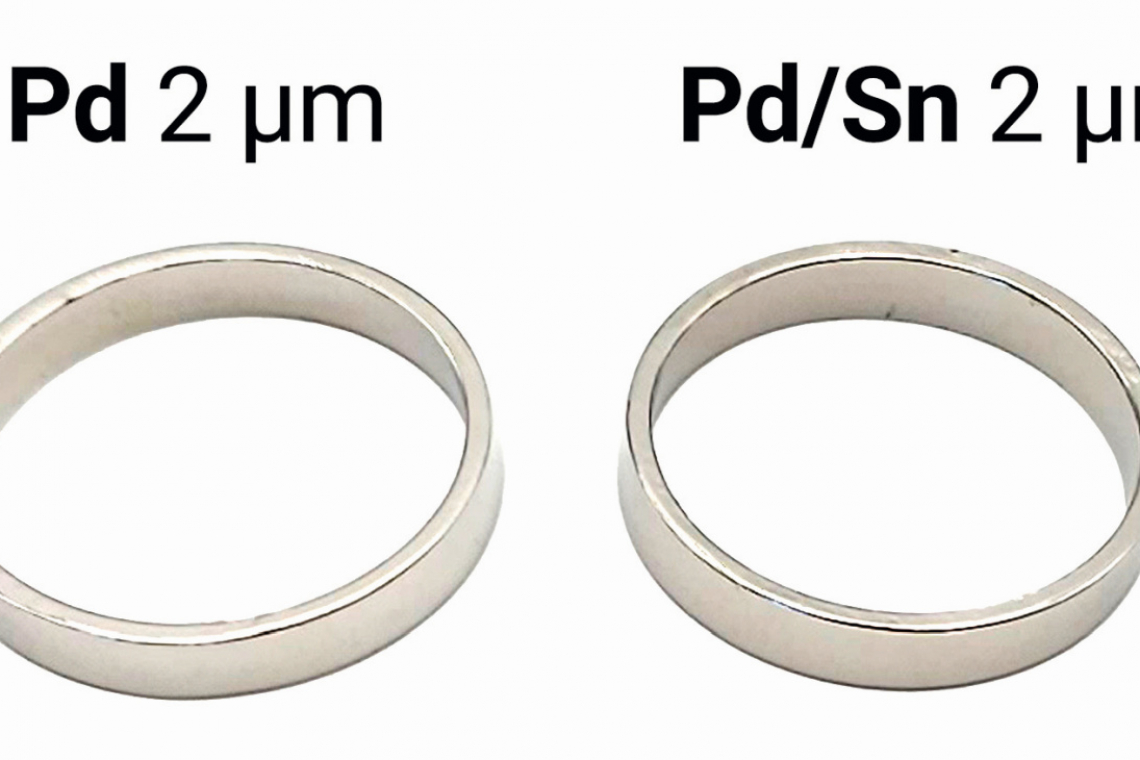
In the jewelry industry and in technical applications, palladium layers can be used as a functional layer. Furthermore, palladium often takes on the function of a barrier layer in demanding layer sequences in order to minimize intermetallic diffusion and prevent or delay corrosion (the basis of corrosion) of base layers or the base material [1, 2]. With Pallega blend Sn, a palladium-tin alloy bath was developed which produces high-quality metal layers at significantly reduced costs compared to pure palladium.
Introduction
As a platinum group metal, palladium is characterized by high resistance to chemical attack and can form dense layers as a soft, ductile metal. It is suitable as a decorative or functional final layer and also as a barrier layer [1,2,3,7,8].
The relatively high price of the metal has a significant impact on the manufacturing costs of finished parts. Accordingly, alloys with less expensive metals have been developed on the market and are already in use.
However, with higher contents of base alloy partners, the coating properties are negatively influenced, be it easier chemical attacks, brittleness and cracking or insufficient brightness in decorative coatings.
Tin, as a pure element, is soft and ductile, just like palladium. When pure, it forms a passive layer and is thus protected to a certain extent against chemical attack [3,4,5]. This metal was selected as an alloying element for extensive experiments.
History and use of palladium[5]
- Discovery in 1803 by William Hyde Wollaston.
- Like gold, pure palladium is a soft and pliable metal. Electroplated palladium is harder and more brittle than metallurgically produced palladium.
- Large quantities of the metal are used in the manufacture of exhaust catalytic converters. Various other applications in electronics, fuel cells, jewelry, ...
- When alloyed with gold and silver, palladium is used for jewelry and watches (white gold).
- Palladium-nickel alloys, e.g. 80 % Pd, 20 % Ni, are well-known and widespread; palladium-cobalt alloys behave similarly, but are not used as frequently.
- Palladium-gold, palladium-silver, palladium-indium and palladium-iron alloys are also known [3, 4, 7, 8].
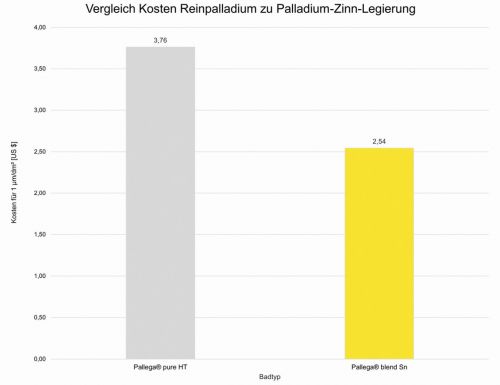 Fig. 1: Cost reduction of approx. 30 % compared to pure palladium with the same layer thickness. IWG Ing. W. Garhöfer GmbH offers both electrolytes based on pure palladium (Pallega pure HT) and Pallega blend Sn based on palladium-tin-nickeland cobalt as alloy components are problematic due to their physiological properties [9], however, and are now dispensed with in many applications. On the one hand, dusts of the metal salts (or aerosols from electroplating baths) are harmful to health, and on the other hand, there is a risk of contact allergy for the end user of the finished product, at least in the case of nickel. Elaborate nickel tolerance tests must be carried out on products if there is a possibility of the metal being released in the event of skin contact when used as intended. This applies, for example, to jewelry, writing instruments, glasses or piercings. Palladium-tin alloys are not widely used. There are some publications on basic research and both metals are cited as alloy components in dental alloys - together with other metals.
Fig. 1: Cost reduction of approx. 30 % compared to pure palladium with the same layer thickness. IWG Ing. W. Garhöfer GmbH offers both electrolytes based on pure palladium (Pallega pure HT) and Pallega blend Sn based on palladium-tin-nickeland cobalt as alloy components are problematic due to their physiological properties [9], however, and are now dispensed with in many applications. On the one hand, dusts of the metal salts (or aerosols from electroplating baths) are harmful to health, and on the other hand, there is a risk of contact allergy for the end user of the finished product, at least in the case of nickel. Elaborate nickel tolerance tests must be carried out on products if there is a possibility of the metal being released in the event of skin contact when used as intended. This applies, for example, to jewelry, writing instruments, glasses or piercings. Palladium-tin alloys are not widely used. There are some publications on basic research and both metals are cited as alloy components in dental alloys - together with other metals.
Tin[6]
- Used for jewelry and consumer goods since the Bronze Age (as a copper-tin alloy).
- Tin in the β-modification is a silvery-white, ductile metal. It can recrystallize at temperatures below approx. 13 °C to form brittle, dark grey α-tin (tin plague).
- Currently, around 30 % of the tin produced is used for tinplate production (cold-rolled sheet steel, electroplated with tin). The metal is also used to make metal objects such as pewter jugs, pewter figurines, organ pipes and much more.
- In window glass production, liquid tin with a mirror-smooth surface can be used to pour the glass over the entire surface.
- Other applications include solder with a low melting point (e.g. 63 % Sn, 37 % Pb or 95.5 % Sn, 0.7 % Cu, 3.8 % Ag).
Current cost comparison of palladium and tin
In August 2024, palladium cost approx. 29,000 euros/kg, tin approx. 32 euros/kg. With this comparison, the incentive to use alloys instead of pure palladium is self-explanatory. Since the raw material costs are only part of the manufacturing costs, the difference in the manufacturing costs of the layer structure is not of the same order of magnitude, but a reduction of approx. 30 % can be expected compared to pure palladium (Fig. 1).
Electroplating of palladium-tin alloys
The electrolyte Pallega blend Sn is cyanide-free and works in a pH range of approx. 7-8 and is suitable for both rack and barrel plating. Compared to pure palladium baths, the bath is significantly less sensitive to cyanide carried over from an upstream coating step.
In applications where nickel or cobalt may not be used as alloy components, it is possible to switch to a palladium-tin alloy. Crack-free layers up to 10 μm thick are possible without any problems due to the ductility of the alloy. The optimum concentration of the alloying elements in the bath is 5-7 g/L palladium and 3-6 g/L tin. The alloy is essentially determined by the concentration of the two metals.
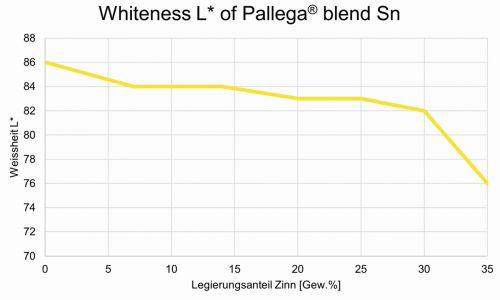
With the bath, alloys with a tin content of up to approx. 30 % can be deposited bright (L* approx. 82-84) and shiny. Above approx. 30 % tin by weight in the alloy, the L* value drops significantly, resulting in a limit for the tin content when used as a decorative coating (Fig. 2).
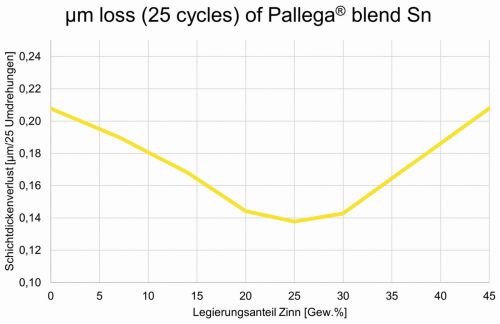
Surprisingly, the palladium-tin alloys were found to be more resistant to abrasion (i.e. less abrasion in the abrasion test: Taber Abraser 5135, 250 g coating, 1000 grit sandpaper, 25 rpm) than electroplated pure palladium. Maximum abrasion resistance was found with an alloy of 75/25 Pd/Sn. As the tin content increases, the abrasion resistance decreases again and deteriorates drastically from a tin content of more than 30 % (Fig. 3).
Scanning electron microscopic investigations
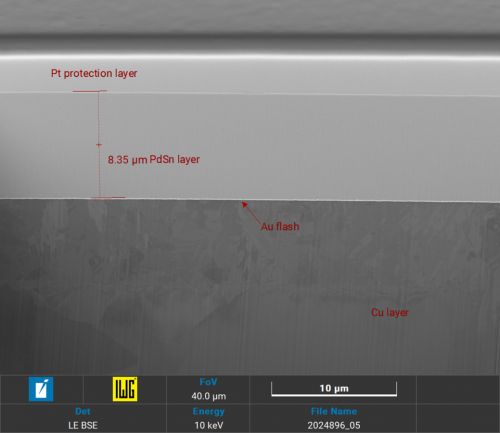
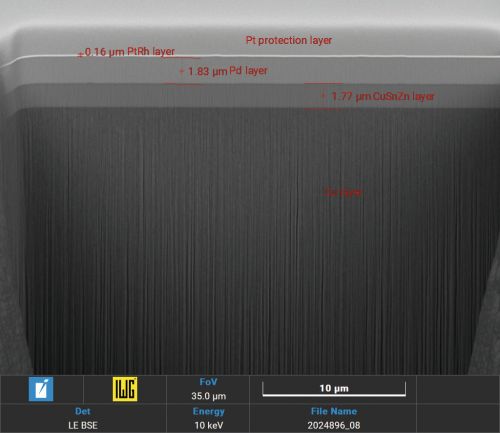
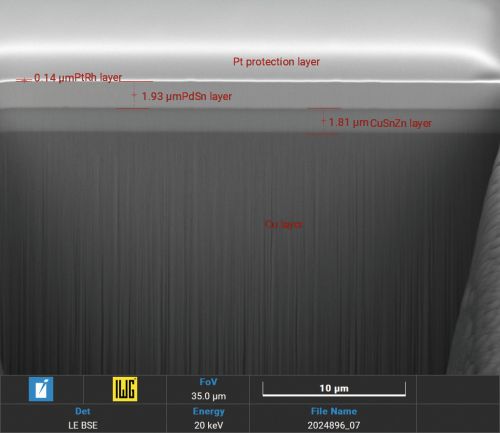
Focussed Ion Beam (FIB) sections with Xe+ plasma in layer structures with palladium-tin alloys show a grain boundary-free, crack-free homogeneous alloy (Fig. 4 and 5).
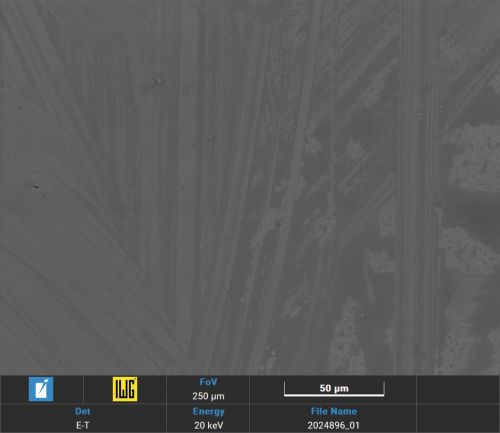
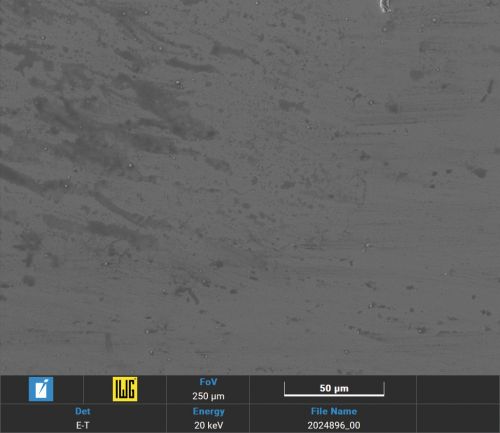
 Fig. 10: Comparison of the Pd and PdSn corrosion tests
Fig. 10: Comparison of the Pd and PdSn corrosion tests
The comparison of the surface of palladium-tin alloys with pure palladium after SO2 corrosion testing [10] shows a similar, only weak attack (Figs. 6, 7, 8, 9 and 10).
Summary
A bath developed for the electrodeposition of palladium-tin alloys is available for the production of decorative coatings and barrier layers. The properties of the alloy are almost the same (brightness) or improved (abrasion resistance) compared to pure palladium. It is not necessary to use questionable nickel or cobalt as an alloying metal. The alloy is ductile and does not tend to crack. The corrosion resistance is more than sufficient for most applications.
Literature
[1] Modern Electroplating, Fifth Ed., 2010
[2] Kaiser, H.: Schriftenreihe Galvanotechnik und Oberflächenbehandlung, Edelmetallschichten, 1st edition. Bad Saulgau: Eugen G. Leuze Verlag
[3] Bogenschütz, A. F.; George, U.: Galvanic alloy deposition and analysis, 2nd edition. Bad Saulgau: Eugen G. Leuze Verlag
[4] Jordan, M.: Die galvanische Abscheidung von Zinn und Zinnlegierungen, 1993, Bad Saulgau: Eugen G. Leuze Verlag
[5] Holleman, A. F.; Wiberg E.: Lehrbuch der Anorganischen Chemie, 102nd edition, 2007, deGruyter, Berlin, pp.1722-1725
[6] Holleman, A. F.; Wiberg E.: Textbook of Inorganic Chemistry, 102nd edition, 2007, deGruyter, Berlin, pp.1002-1007
[7] K.M. Chow, W.Y. Ng, L.K. Yeung, Barrier properties of Ni, Pd and Pd-Fe for Cu diffusion, Surface and Coatings Technology, Volume 105, Issues 1-2, 1998, Pages 56-64
[8] Baumgärtner, M. E., & Gabe, D. R. (2000). Palladium-Iron Alloy Electrodeposition. Part II Alloy Plating Systems. Transactions of the IMF, 78(2), 79-85
[9] https://echa.europa.eu/de/substance-information ... Current substance information for chemicals
[10] DIN EN ISO 22479:2022-08, Corrosion of metals and alloys - Test with sulphur dioxide in a humid atmosphere (fixed gas volume method) (ISO 22479:2019)