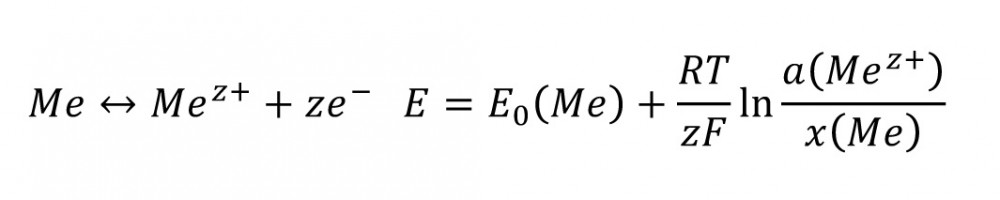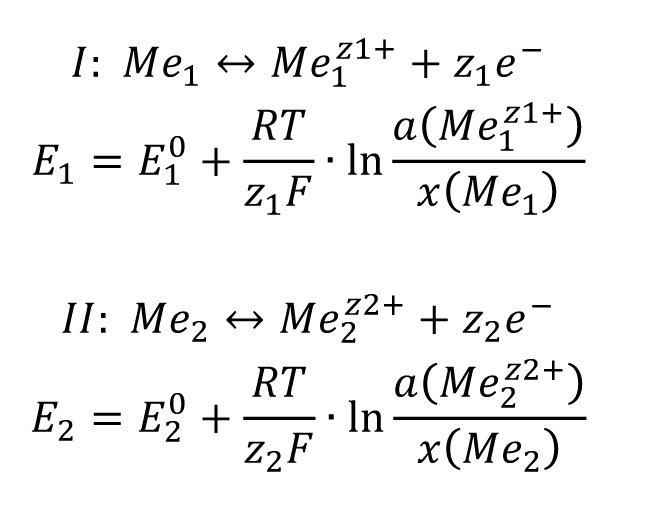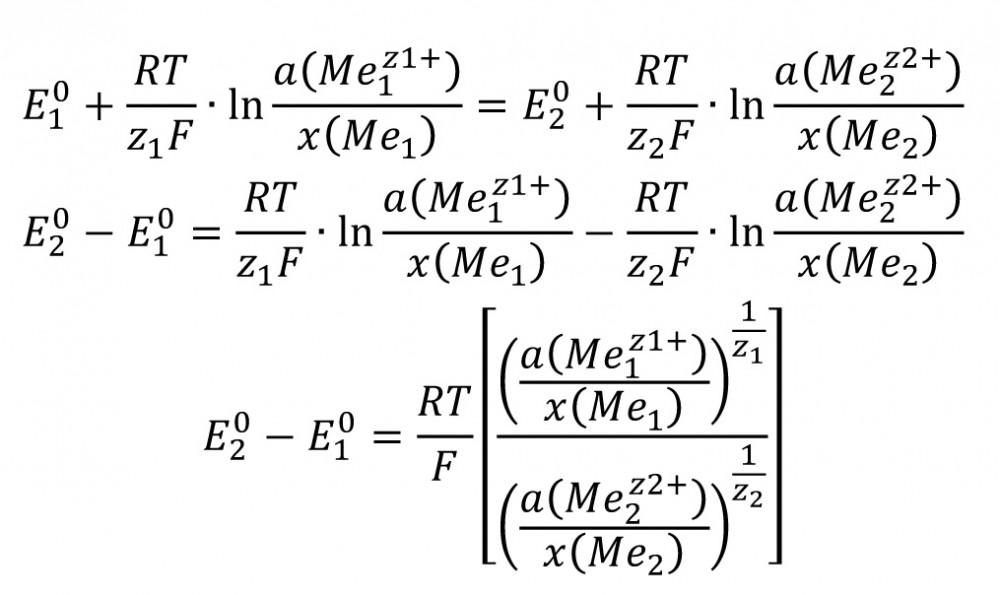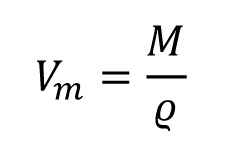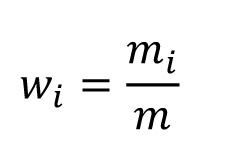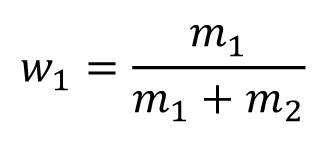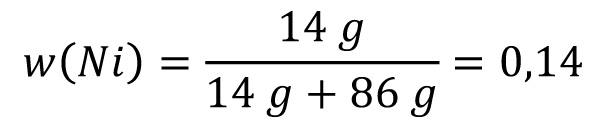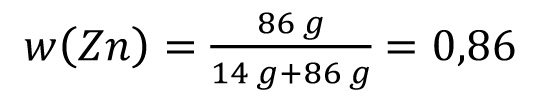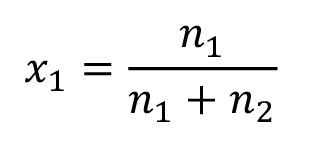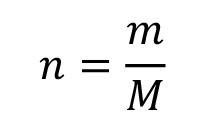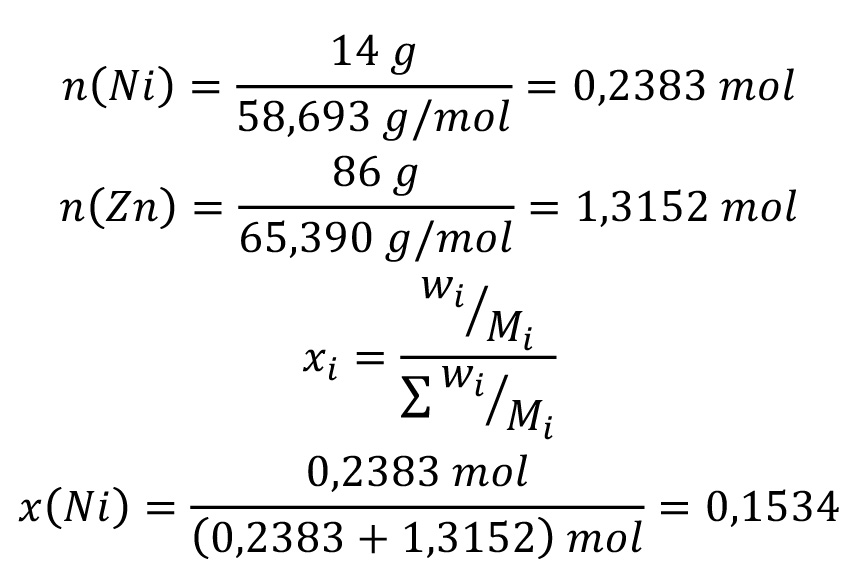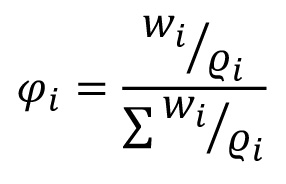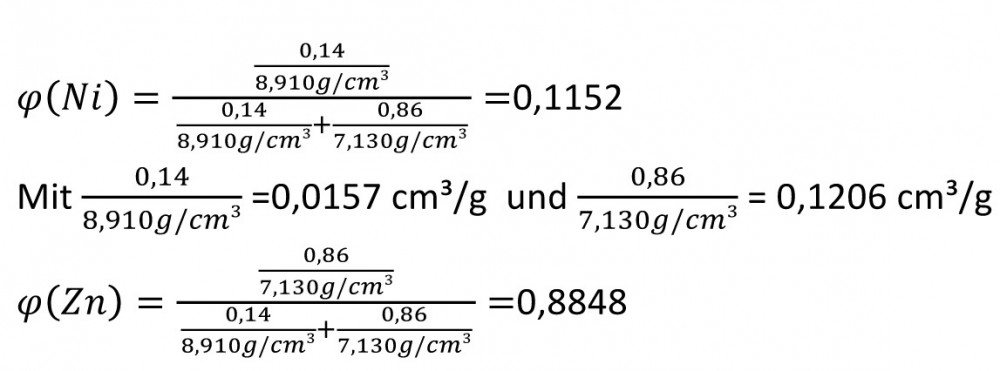One property of the automotive alloy ZnNi14 is to push the mixing potential as close as possible to the potential of the steel. This greatly reduces the drive for corrosion and the corrosion current density.
The potential must not become more positive than the latter in order to maintain the cathodic protection of the coating. On the other hand, it should be as high as possible in order to reduce the corrosion rate. At the same time, the alloy has a higher thermal load capacity. The NiO that forms protects the surface. Another advantage is the lower volume of corrosion products compared to zinc. The higher hardness also offers improved wear behavior.
This actually priceless alloy is always used when a simple Zn coating is not sufficient. Two developments over the last 50 years have contributed to this:
1. road salt is used on all roads today.
2. the increasingly compact design of drives leads to higher temperature loads on the components.
The alloy layer is also sustainable. The process can help to meet the challenges of e-mobility. This is because zinc-nickel surfaces enable contacting in the high-voltage range as well as mass feedback and have a positive influence on disintegration wear [3].
A very specific electrode potential applies to every electrode reaction.
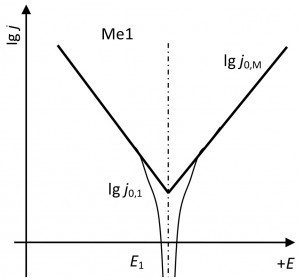 Fig. 1 Current density-potential curve of a metal electrodeThismeans that the potential becomes nobler with increasing concentration of the metal cations in front of the surface a(Mez+) and with decreasing amount of material of the metal concentration in the electrode x(Me).
Fig. 1 Current density-potential curve of a metal electrodeThismeans that the potential becomes nobler with increasing concentration of the metal cations in front of the surface a(Mez+) and with decreasing amount of material of the metal concentration in the electrode x(Me).
If the potential of the electrode deviates from this potential, a current flows through it whose polarization just compensates for the difference. The current density-potential curve is defined and is shown in semi-logarithmic representation in Figure 1 below.
A mixed potential is formed at an alloy electrode [1]. The two current density-potential curves overlap (superposition principle). The deviation from the two normal potentials equalizes according to the above. The potential ratios are as follows:
E1 =E2 inevitably applies at the mixed potential.
The concentrations are determined according to this equation.
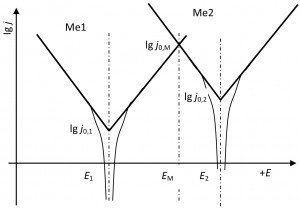 Fig. 2: MPK current density-potential curves of two metal electrodes with mixed potential formationTheresulting exchange current j0, which consists of an equal anodic and cathodic current, results from the potential deviation of the respective individual electrode (Fig. 2).
Fig. 2: MPK current density-potential curves of two metal electrodes with mixed potential formationTheresulting exchange current j0, which consists of an equal anodic and cathodic current, results from the potential deviation of the respective individual electrode (Fig. 2).
Facts about this:
- The mixed potential is always between the two output potentials.
- The exchange current density of the mixed electrode is always greater than the output exchange current densities.
- The mixed potential is always closer to the potential of the electrode with the higher exchange current density.
- The anodic part of the mixed exchange current density is supplied by the metal with the more negative potential.
- The cathodic part of the mixed exchange current density is supplied by the metal with the higher potential.
- Can this lead to depletion of the less noble metal and shift the potential to more noble values?
Yes, the dezincification of brass, for example, is well known.
Unfortunately, it is also incorrect to use the exchange current densities of the base metals. You have to reduce them according to the surface fraction. The surface fraction corresponds to the volume fraction. Accordingly, the respective exchange current density in an alloy decreases compared to the pure metal.
The density results from the ratio of molar mass and molar volume. The molar volume can therefore be determined as follows:
The following must be determined for the exchange current densities of the alloy: Mass fraction: w; mass fraction x; volume fraction: ϕ.
Mass fraction:
For a binary alloy
For 100 g ZnNi14 applies:
86 g Zn + 14 g Ni
Correspondingly:
Amount of material x
With
For 100 g:
Volume fraction ϕ
Density ς(Ni) = 8.910g/cm3; ς(Zn) = 7.130g/cm3;
The following can be assumed for the exchange current densities in the sulphuric acid corrosion medium [4]: j0,Ni= 1*10-7 A/dm2; j0,Ni= 1*10-3 A/dm2;
This gives the actual exchange current densities.
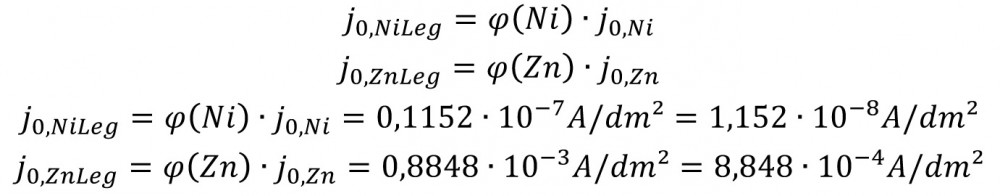 The exchange current densities have decreased according to the alloy composition, and by different factors:
The exchange current densities have decreased according to the alloy composition, and by different factors:
Ni: 1.152*10-8/1*10-7 = 0.1152
Zn: 8.848*10-4/1*10-3 = 0.8848
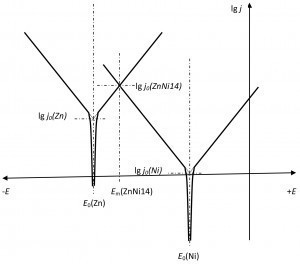 Fig. 3: Current density-potential ratios on ZnNiTheexchange current density of nickel, with its significantly lower concentration, has decreased accordingly.
Fig. 3: Current density-potential ratios on ZnNiTheexchange current density of nickel, with its significantly lower concentration, has decreased accordingly.
- The exchange current density of the metal with the lower concentration decreases more than that with the higher concentration.
- As a result, the mixed potential shifts (more) towards the metal with the higher content.
In the case of ZnNi14 to zinc.
The following ratios apply to the zinc-nickel alloy ZnNi14 (Fig. 3)
- The mixed potential lies between the potentials of nickel and zinc.
- The exchange current density is significantly greater than that of nickel and zinc.
- The mixed potential is closer to the potential of zinc.
- The anodic part of the exchange current density is supplied by the zinc.
- The cathodic part of the exchange current density is supplied by nickel.
- A depletion of zinc leads to a reduction in the exchange current density of the zinc and thus to a potential shift in the direction of the nickel potential.
- The exchange current density of nickel decreases more than that of zinc as the alloy is formed.
- The latter leads to a potential shift in the direction of the zinc compared to a construction from the individual current density-potential curves.
From a dynamic point of view, the nickel content in the alloy increases with corrosion. The surface of the layer becomes more noble, i.e. the potential shifts in the direction of the nickel. This is particularly favorable under thermal stress, as the surface is then protected by a NiO layer.
Special potential problems occur when intermetallic phases form. They do not have a purely metallic bond, but also contain other types of bond. In any case, the bonds are stronger, i.e. more energetic than the simple metallic bond. Due to the two (or more) types of ions, it is a mixed electrode, but it is more noble than a simple mixed electrode of the same composition. This "mixed potential can only be determined by establishing the intermetallic bond and recording the current density-potential curve.
Two intermetallic compounds are formed in the nickel-zinc system, Ni5Zn21 and NiZn8 [2] Their greater stability is reflected, among other things, in the melting point, which is 881 °C for Ni5Zn21.
Literature
[1] Textbook of electrochemistry, Unruh, J.N.M., Leuze Verlag, 1st edition, Bad Saulgau, 2013
[2] Crystal Structure of Metals and Alloys, Handbook Structure and Properties of Metals and Alloys, vol. 1 (Russian), ed. L.N. Larikov, Publ. Naukowa Dumka, Kiev, 1986
[3] https://holzapfel-group.com/oberflaechenverfahren/korrosionsschuetzende-beschichtungen/zn-ni-zink-nickel.html
[4] Table book electroplating technology, Unruh, J.N.M., Leuze Verl. Bad Saulgau, 8th ed., in print