Steadily growing demand for electronic assemblies has ensured stable utilization of electronics production capacities for decades. However, this means that more and more residues are produced in the production process and are deposited in the soldering systems. These condensate residues not only impair the soldering process itself, which is why the causes and mechanisms of condensate formation are discussed in more detail here.
Condensate residues affect heat distribution because they insulate areas in the soldering system that are to be heated or cooled. They can also clog extraction lines and thus affect the amount of heat, which is critical for an optimal soldering process. They are also corrosive and damage machine components in the long term. Condensate residues also affect the quality of the finished PCBs. Depending on what has been processed and in what combination, certain residues can remain on the PCB after cleaning and cause negative long-term effects.
Residues are problematic
Cleaning systems to remove condensate residues takes time. This time is often no longer available in the production process. If a lot of condensate residue builds up, the cleaning intervals must be shortened. Above all, the residues pollute the environment as waste. Contact with condensate residues can also be harmful to health and trigger allergic reactions. It is therefore necessary to deal with condensate in detail and understand the mechanisms of its formation.
Hardly anyone is interested in the details of the amber-colored condensate residues in the soldering systems, which become continuously darker as the temperature increases. Needle-shaped crystalline formations also occur during the maintenance process, usually on the inner walls of the extraction pipes - in areas with a large temperature gradient. This article deals with the cause of condensate formation and the mechanisms responsible for it. In this way, technologies and processes can be developed to minimize condensate formation from the outset.
A complex process
The formation of condensate in the soldering process is a complex process. This is because outgassing, chemical reactions and condensation of the volatile low-molecular components play a major, but not exclusive, role. The production environment, temperature window for condensate formation and the presence of suitable reaction partners are just some of the many other factors that contribute to condensate formation. Effects such as a consistent failure to observe the laws of thermodynamics in the geometry of the extraction lines in a soldering machine or the use of materials with a catalyst effect also play an important role(Fig. 1). The aim of this article is therefore to provide a basic understanding of the formation mechanisms of condensate in the soldering process and thus make a meaningful contribution to its minimization.
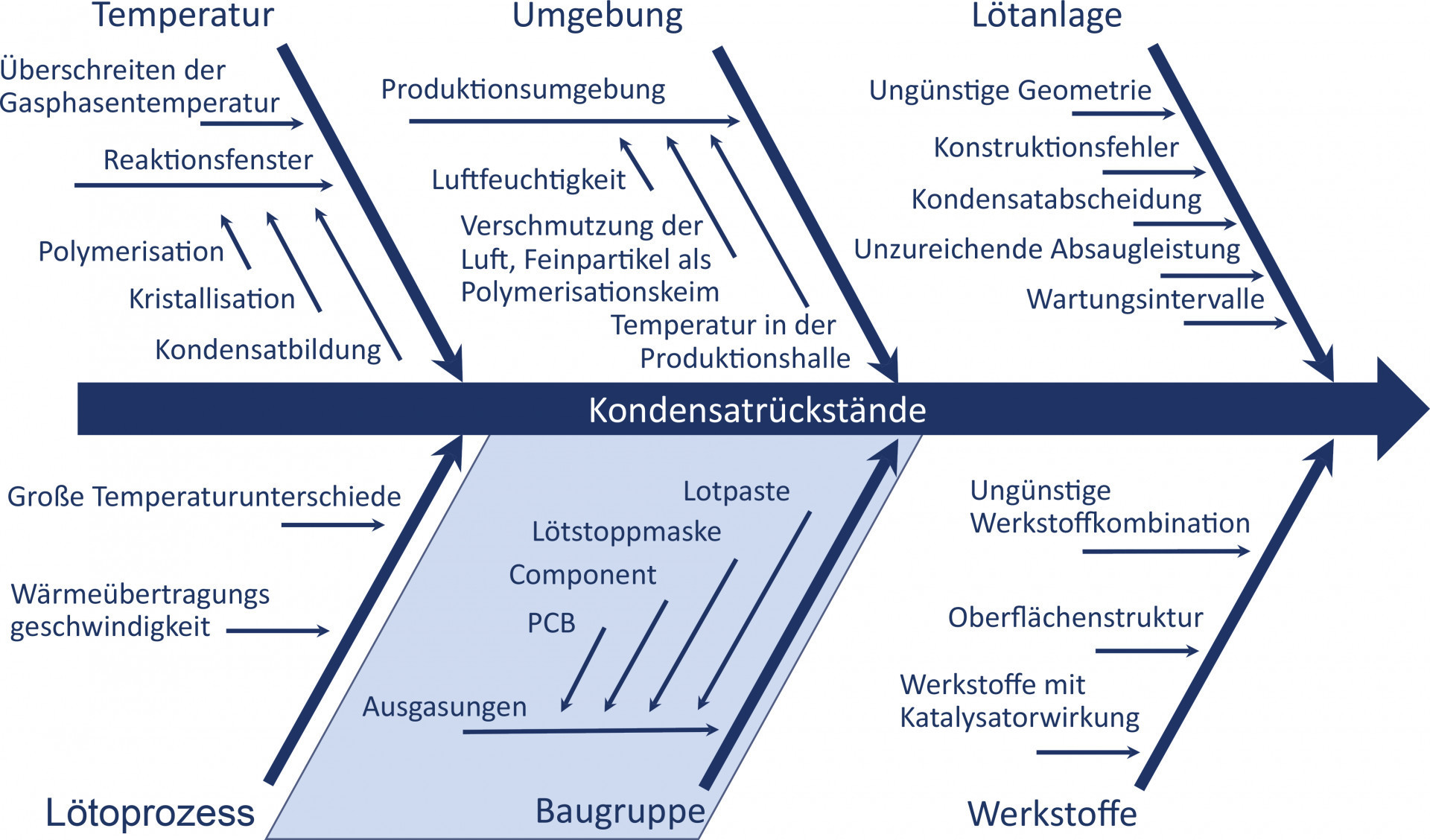 Fig. 1: Causes of condensate formation
Fig. 1: Causes of condensate formation
Condensate formation
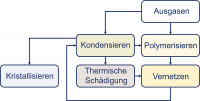 Fig. 2: Condensate formationSinceeven the best quality of the individual components cannot prevent all components from reacting chemically with each other during the manufacturing process, not only the solder paste but also the PCB substrate, the solder resist and the housings of the components begin to outgas in the soldering system. These gaseous molecules can react with each other and form larger molecules. If the influence of temperature is added and suitable conditions are present, polymerization and cross-linking take place(Fig. 2 and 3).
Fig. 2: Condensate formationSinceeven the best quality of the individual components cannot prevent all components from reacting chemically with each other during the manufacturing process, not only the solder paste but also the PCB substrate, the solder resist and the housings of the components begin to outgas in the soldering system. These gaseous molecules can react with each other and form larger molecules. If the influence of temperature is added and suitable conditions are present, polymerization and cross-linking take place(Fig. 2 and 3).
Polymerization
In order to understand condensate formation, formation mechanisms from polymer chemistry must be used. The polymerization process describes the growth of an organic molecule (monomer) into longer molecular compounds (polymers). Different organic molecules can polymerize to form long polymer chains. The prerequisites for polymerization are reactive bonds, suitable polymerization partners and favourable polymerization conditions (temperature and reaction time). The soldering process provides all of this. There are different temperatures, a continuous supply of suitable reaction partners is ensured, which usually have the reactive bonds - and if not, the thermal damage ensures that these can be formed. Figure 4 shows an example of a polymerization reaction of abietic acid from the solder paste.
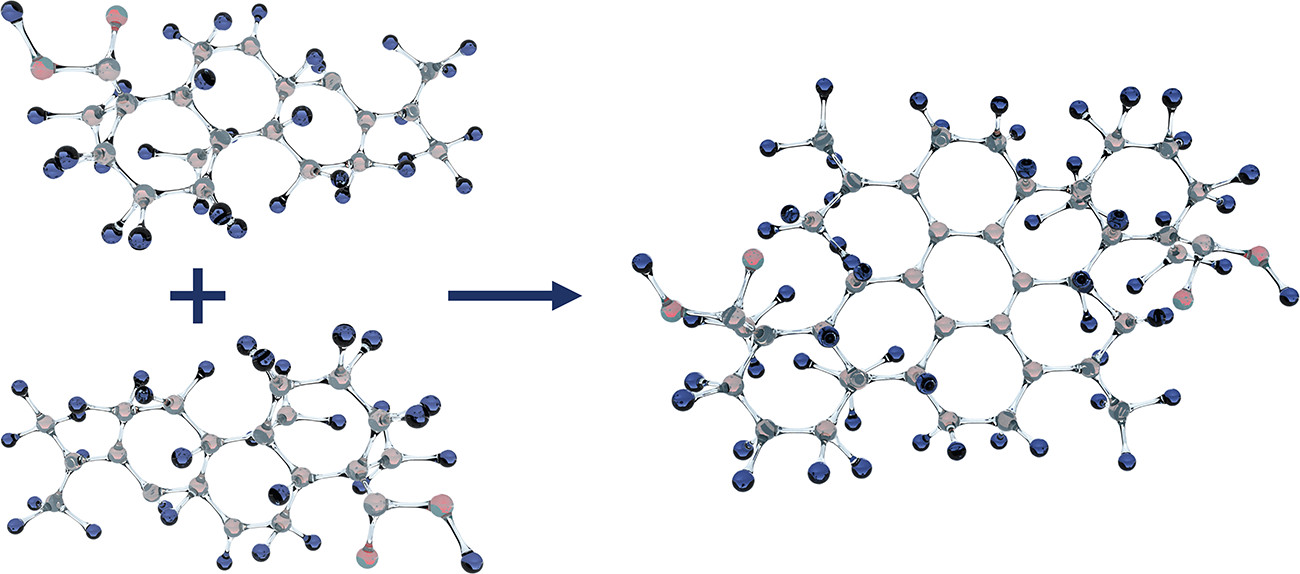 Fig. 3: Formation of a dimer from two monomers of abietic acid (from the solder paste)
Fig. 3: Formation of a dimer from two monomers of abietic acid (from the solder paste)
Cross-linking
Cross-linking is the molecular linkage between the individual polymer chains formed. The degree of cross-linking significantly determines properties such as strength, dimensional stability and chemical and thermal resistance(Fig. 5).
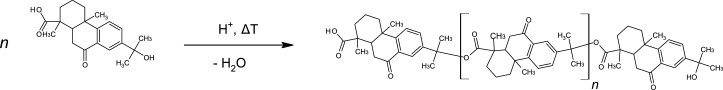 Fig. 4: Formation of a 5-hydroxy-7-oxodehydroabietic acid polymer by polycondensation of abietic acid (from the solder paste)
Fig. 4: Formation of a 5-hydroxy-7-oxodehydroabietic acid polymer by polycondensation of abietic acid (from the solder paste)
The high temperature in the soldering systems favors the cross-linking reactions, since in addition to the continuous release of the reactants, thermal damage to the existing, already cross-linked layer also plays a major role. At high temperatures, the molecular chains of the already cross-linked layer are thermally damaged and new reactive groups are formed which are available for further cross-linking reactions. It is therefore not only the release of reactive low-molecular compounds that is responsible for the degree of crosslinking, but also the duration of the thermal effect on the already polymerized condensate layer. In practice, this continuous post-crosslinking leads to increased cleaning requirements for the soldering systems, as this increases both the chemical and mechanical resistance of the condensate layer.
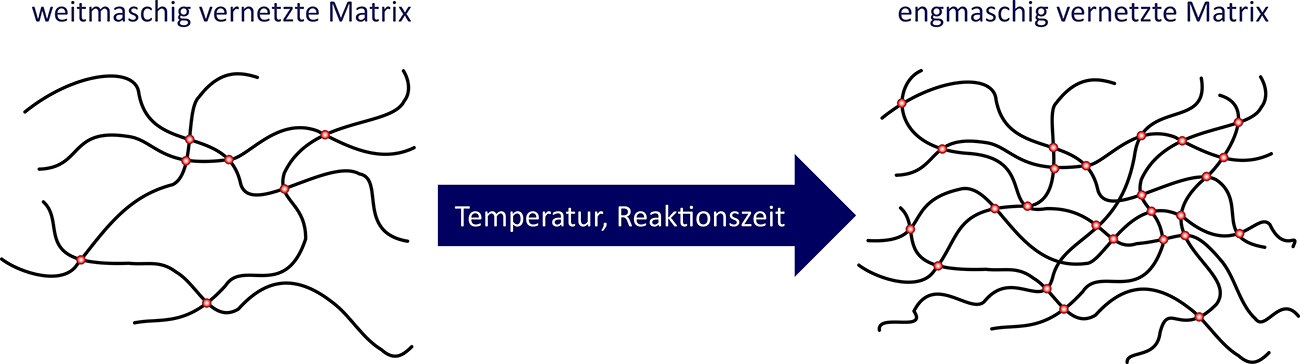 Fig. 5: Wide-mesh cross-linking vs. close-mesh cross-linking
Fig. 5: Wide-mesh cross-linking vs. close-mesh cross-linking
Condensation process
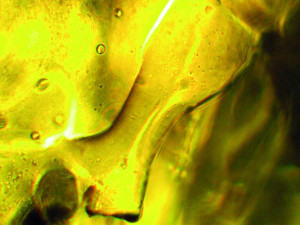 Fig. 6: Cross-linked condensate from a reflow system (400x magnification)The reaction products condense as soon as the ambient temperature has cooled down sufficiently. The larger and bulkier the macromolecules are, the higher the temperature at which they condense. Smaller and lighter macromolecules, on the other hand, condense at a lower temperature. If favorable conditions prevail, such as a suitable temperature window, sufficient time for growth processes and suitable building blocks are available, the formation of crystalline structures takes place(Fig. 7). These can grow from the unreacted low-molecular compounds from the circuit board substrate or from the components of the solder resist. Crystallization processes can also start from the reaction products of the flux.
Fig. 6: Cross-linked condensate from a reflow system (400x magnification)The reaction products condense as soon as the ambient temperature has cooled down sufficiently. The larger and bulkier the macromolecules are, the higher the temperature at which they condense. Smaller and lighter macromolecules, on the other hand, condense at a lower temperature. If favorable conditions prevail, such as a suitable temperature window, sufficient time for growth processes and suitable building blocks are available, the formation of crystalline structures takes place(Fig. 7). These can grow from the unreacted low-molecular compounds from the circuit board substrate or from the components of the solder resist. Crystallization processes can also start from the reaction products of the flux.
The theoretical consideration of condensate formation is the first step towards a better understanding of the elements and parameters involved and their interaction with each other in the soldering environment. Condensate is more than just a condensed residue from the flux or solder resist on the walls of the soldering equipment. Condensate formation must take into account volatile components of the PCB substrate, solder mask and solder paste flux. They can all react with each other during the soldering process and thus determine condensate growth, the amount of condensate and its composition.
 Fig. 7: Crystalline condensate residue from a reflow process (100x magnification)
Fig. 7: Crystalline condensate residue from a reflow process (100x magnification)
Understanding the formation, reaction possibilities, cross-linking and crystallization helps to take appropriate measures. For example, certain reactions can be prevented or inhibited by changing the temperature outside the process in order to slow down the polymerization process. Crystallization processes can be slowed down by targeted extraction of the atmosphere at points with preferential outgassing of certain low-molecular compounds, as only a small quantity of the corresponding reaction partners is available for a crystallization process.