It has long been known that metals in waste water are lost valuable materials that have a direct impact on production costs. However, costs can be reduced by optimizing electroplating production costs, i.e. automation, process bath maintenance and improved yield.
However, further production cost optimization is still possible. The coating metal used should be on the components and not end up as waste in waste water.
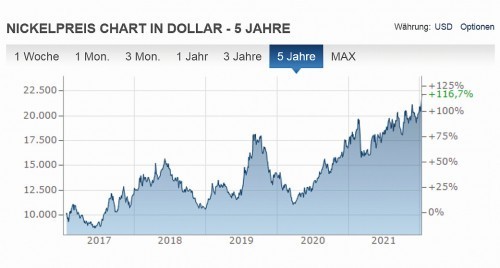
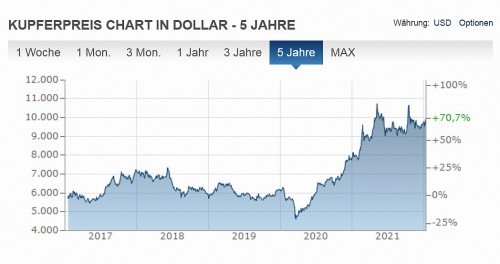
In particular, the price development of coating metals plays a very important role here. This can easily be seen from the price trend for copper and nickel over the past five years (see Fig. 1 and Fig. 2).
This metal recovery from rinsing water is carried out in various processes, whereby a distinction must be made between genuine recycling and "downcycling". Here, a separation of the wastewater streams must be ensured - e.g. nickel-containing wastewater, copper-containing wastewater.
Methods of recycling As metal mono-sludge
This is certainly the most widely used method of recycling. Conventional hydroxide precipitation produces a so-called mono-sludge, which can be dewatered to a dry substance content of up to 35%.
The metal content is only 5-8% of the pressed sludge. The recovery of valuable materials is correspondingly low, as the processing of the sludge is also associated with high costs. This is typical "downcycling".
As metal
Here, the metal is concentrated and purified using ion exchange technology. The resulting regenerate with a metal content of 40-60 g/l is then fed into an electrolysis process in which the metal is deposited.
The payback is essentially based on the daily metal scrap price quoted on the stock exchange.
If the recycled metal is used directly in the electrolysis process, this is direct recycling. However, the "scrap" is usually returned to the metal smelters, which corresponds to "downcycling".
As an electrolyte
With various electrolytes, it is possible to concentrate rinsing water using an evaporator (Fig. 3) to such an extent that the concentrate can be reused to supplement the electrolyte after subsequent cleaning. This has already been used successfully in cyanide copper baths. However, the corresponding bath additives such as brighteners, levelers etc., as well as their decomposition products, play a limiting role here, as they are also concentrated.
As a metal salt solution
The metal is concentrated and purified using ion exchange technology. The AGW Antech Gütling regeneration technology purifies the resulting regenerate with a metal content of approx. 60 g/l in such a way that the impurities it contains are reduced to a minimum.
By means of evaporation technology, this regenerate can be concentrated even higher so that a direct metal salt recycling into the electrolyte can take place.
This direct recycling achieves the highest payback of the processes listed.
Application in electroplating technology
After intensive preliminary discussions with AGW Antech Gütling, a renowned, medium-sized electroplating company opted for a complete nickel recycling system to return the nickel that had been dragged out to the plating process. The target here was a nickel concentration of 210 g/l. Such a value can only be achieved using a combination process, as there are limits to concentration through ion exchange. The regenerate concentration is over 60 g/l nickel. Consequently, the regenerate must be further concentrated by a factor of 1 : 3.5 up to the target concentration using evaporation technology. As part of preliminary tests, the ion exchange process step was examined with regard to the following objectives:
- Selection of a suitable ion exchange resin with the highest possible usable volume capacity.
- Optimized, complete loading of the ion exchanger until breakthrough of the working column. With 100% breakthrough of the first column, 2 to 3 columns are still available for virtually complete denitrification of the rinsing water. The exchangers are operated via automatically controlled valves in a series connection. After the nickel has been largely removed, the wastewater can be fed to the final neutralization with gravel filter and selective exchanger without chemical-physical wastewater treatment.
- Optimized acid regeneration with regeneration blending. In this regeneration process, a significantly different HCl fresh acid concentration is used compared to fresh water treatment. Each proportionate volume of fresh acid is used several times within a regeneration cycle, whereby the volume of the previous regeneration step is collected and passed back over the column in the subsequent step. This results in a successive enrichment of nickel in the partial quantities of regenerating acid and ultimately in the regenerate, up to the maximum achievable final concentration. The final regeneration step is always carried out with fresh acid to ensure complete regeneration of the resin bed.
- Conditioning of the resin. To optimize the usable volume capacity of the resin, it must be conditioned.
- Rinsing water produced during regeneration: The rinsing water produced during regeneration is returned to the recycling process as part of the recovery process in order to keep the nickel it contains in the cycle.
This and other process engineering steps are used to optimize the nickel recovery rate. Based on the preliminary tests, as well as through optimization measures during commissioning, the nickel concentration in this practical example was optimized to over 60 g/l.
Concentration to a target nickel concentration of 210 g/l
As described in the requirement profile, a nickel concentration of 210 g/l was to be achieved in the product. This necessitated the use of an evaporator. The following requirements must be met for this concentration in the evaporator:
- In addition to nickel, the regenerate also contains a high chloride content, i.e. corrosion potential on stainless steel. For this reason, neutralization of the regenerate is unavoidable. However, the regenerate is only slightly acidic due to the optimized regeneration. In addition, there is almost no free hydrochloric acid.
- Evaporator type: A heat pump evaporator (Fig. 4) with a scraper system and boiling temperature of approx. 35 °C was selected for concentration, which means a lower corrosion potential than higher temperatures. The installed scraper system also avoids the crystallization of nickel chloride on the boiler and thus prevents a collapse of the heat transfer at solution densities of up to 1.4 g/cm3.
- Evaporator material: Durable stainless steel was used as the evaporator material. Alternatively, titanium is conceivable, i.. i.e. significantly reduced risk of corrosion.
Conclusion:
The advantages of metal or electrolyte recycling (usually Cu and Ni) in electroplating technology are obvious compared to conventional chemical-physical wastewater treatment: every liter of metal-containing electrolyte discharged generates costs for the operator due to the consumption of precipitation chemicals (caustic soda and/or milk of lime), sludge disposal (hazardous waste), resharpening of the bath electrolyte using metal salts, personnel costs for plant operation (e.g. emptying the filter press, sample testing) and analytical costs.(e.g. emptying the filter press, sample testing) as well as analytical regulatory and self-monitoring.
The advantage of early cooperation between the user, the electroplating plant manufacturer, the chemical supplier and the wastewater and recycling plant manufacturer is particularly evident when planning new plants. This cooperation includes the fine-tuning of the rinsing technology and the low-carryover goods transport, the bath chemistry and the possible recovery techniques.
Photos: AGW Antech Gütling