Galvanizing is a proven coating for steel surfaces to protect against corrosion. For temporary protection against loss of gloss and white rust formation, an air-drying, water-based dip coating is applied directly after galvanizing. As part of a publicly funded project, a research institute in Jena has developed a bio-based alternative in collaboration with an industrial partner. Will it be possible to use renewable raw materials for corrosion protection in the future? The following article will take a closer look at the potential of these coatings based on special sugar molecules.
Corrosion protection of steel surfaces through galvanization
Steel surfaces are subject to corrosion without protection. A coating with a thin layer of zinc can largely prevent this. The galvanizing process is carried out using different methods. Common galvanizing processes are hot-dip galvanizing (hot-dip galvanizing), electrogalvanizing (electrolytic galvanizing), mechanical galvanizing and spray galvanizing. Only hot-dip galvanized samples were used in this work. The zinc coating is applied during this process by immersion in a molten zinc bath at a temperature of approx. 450 °C. As zinc is chemically less noble than iron, it releases electrons in a humid environment and goes into solution, "sacrificing" itself for the chemically more noble iron (Fig. 1). This process begins immediately after the molten zinc solidifies in the air and is particularly pronounced when stored outdoors. The natural process of patina formation provides additional passive protection. The stable and firmly adhering zinc patina is formed by the simultaneous action of atmospheric oxygen, water from precipitation or air humidity and the carbon dioxide contained in the air. As a result of the natural protective layer formation, the surface becomes matt gray and gradually loses its shiny metallic appearance. In addition, the corrosion of the zinc slows down considerably.
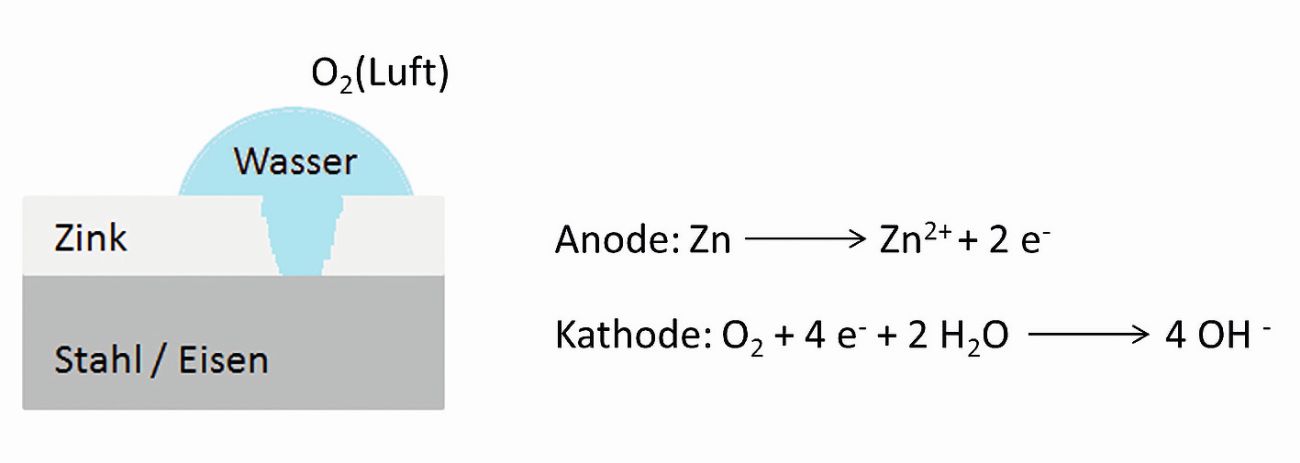 Fig. 1: Principle of cathodic corrosion protection of steel by galvanizing
Fig. 1: Principle of cathodic corrosion protection of steel by galvanizing
White rust formation
The dry storage of galvanized building elements in accordance with the material is possible with practically no time limit. The patina formation described above occurs as a result of the natural effect of humidity and carbon dioxide from the air. As a rule, water without aggressive substances does not damage galvanized flatware if it can evaporate or run off again quickly and there is access to air. The surface only becomes matt gray over time as a result of the increasing natural top layer formation and loses its shiny metallic appearance. However, if water, especially condensation, acts on a galvanized surface over a longer period of time without carbon dioxide ingress from the air, a whitish to light grey, powdery, voluminous zinc corrosion product forms instead of the firmly adhering patina (Fig. 2). This corrosion product is known as white rust. White rust consists mainly of zinc hydroxide, a small proportion of zinc oxide and some zinc carbonate (patina) [1]. However, white rust adheres poorly and therefore does not provide any protection. Typical white rust-promoting conditions arise, for example, when moisture penetrates between stacked metal sheets, is held there by capillary action and hardly any air can enter. Slight white rust is easy to remove and does not affect the workpiece's workability or corrosion protection. However, persistent conditions that promote white rust can lead to the formation of red rust over time and thus to the local destruction of the corrosion protection. Heavy white rust infestation should therefore be removed if possible. In addition, the voluminous corrosion products can bind moisture well and prolong the conditions that promote white rust. To remove white rust, the entire surface is thoroughly treated with hard nylon brushes and then rinsed with warm water. Further treatment depends on the extent and degree of damage. In the event of a significant reduction in the zinc coating thickness, it may be necessary to restore the original corrosion protection by applying suitable coatings. In the case of severe white rust infestation, the uniform appearance of the zinc surface can usually not be fully restored.
Temporary coatings and measures for long-term corrosion protection of zinc
According to the state of the art, an air-drying, water-based dip coating is applied directly after galvanizing for temporary protection. The aim is to maintain the surface gloss and prevent white rust as far as possible. This 3-10 micrometer thick protective layer wears off within the first six months after application. Oil or other protective coatings applied during processing can also delay the corrosion processes described. The question of material-appropriate storage is largely reduced to preventing storage conditions that promote the formation of white rust.
Duplex systems are mainly used for the long-term corrosion protection of galvanized goods. According to DIN EN ISO 12944-5, this is a "corrosion protection system consisting of galvanization in combination with one or more subsequent coatings". Both corrosion protection systems complement each other perfectly. Duplex systems have become indispensable in many areas of corrosion protection for steel structures, such as in the construction industry, road traffic or energy supply. The term duplex covers both liquid and powder coatings on galvanized substrates. Powder coating coaters are faced with major challenges due to the dip coating used for temporary protection after galvanizing, as the pickling process required for pre-treatment is prevented. Particularly in humid environments, the coating with powder coatings very quickly leads to qualitative defects in the form of insufficient adhesion. To ensure the required surface quality, the temporary protection must first be removed at great expense using mechanical and chemical pre-treatment methods prior to pickling. Depending on the duration and the conditions prevailing during storage, measures may also be required to remove white rust and refurbish the surface.
Corrosion protection based on renewable raw materials
In recent years, there has been an increasing focus on the development of more environmentally friendly and sustainable coating processes in the field of surface protection. One of the main areas of research is the development of cost-effective coatings based on polysaccharides. The polymer class of polysaccharides, also known as polysaccharides, is widespread in nature. They consist of a large number of monosaccharide units that are linked to each other via a chemical glycosidic bond (Fig. 3).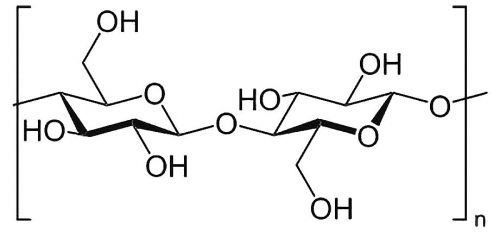 Fig. 3: Structural formula of the polysaccharide cellulose
Fig. 3: Structural formula of the polysaccharide cellulose
Due to their hydrophobic character and thermoplastic properties, modified polysaccharides based on starch and cellulose are particularly suitable for temporary corrosion protection. The dispersibility and solubility of polymer powders in water, such as starch esters and cellulose carbamates, is also known from the literature [2]. The activities of the Jena-based industrial research institute Innovent focus primarily on carbon and fatty acid ester-modified derivatives. Corresponding starch esters can be produced efficiently and with a wide range of properties, such as melting point, hydrophobicity, transparency and elasticity, from starch and an activated carboxylic acid in an imidazole melt (Fig. 4) [3].
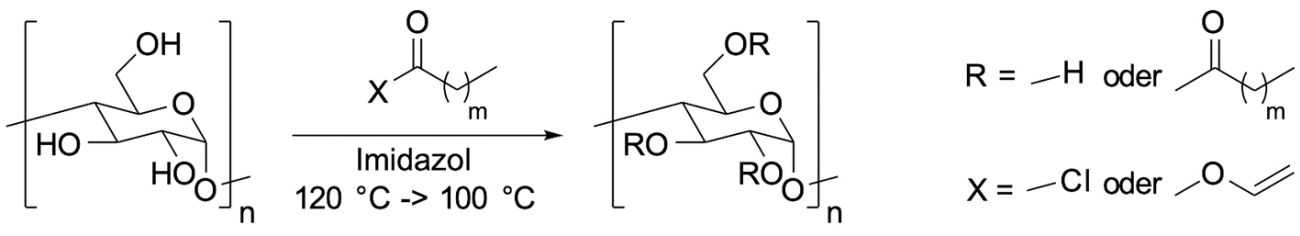 Fig. 4: Synthesis scheme of a starch fatty acid ester in imidazole melt
Fig. 4: Synthesis scheme of a starch fatty acid ester in imidazole melt
A patent (US 8277554B2) has been published jointly with the Friedrich Schiller University Jena. In connection with these starch derivatives, Innovent's focus is on powder coating analogs. These have been used, for example, to protect ferrous archaeological finds (Fig. 5) and on an excavator shovel (Fig. 6) [4].
Numerous cellulose esters can already be purchased commercially. Depending on the chemical modification of the side chains in the molecule, the melting behavior of the polymer powder can also be specifically controlled. Based on these prerequisites, Innovent and Bader Pulverbeschichtung GmbH from Aalen have been working on an alternative to conventional temporary corrosion protection products for galvanized components. In the first stage of a joint, publicly funded research project, investigations were carried out into the melting behavior of selected cellulose carboxylic acid esters. Subsequently, the focus was on the production of stable aqueous preparations. At this point, a dispersant and a mixture of ice and water were used in addition to the respective polymer powder. The concentration range of the suspensions produced was between one and ten percent by weight. The polymer powders were ground and sieved in advance using a planetary mill and prior freezing with liquid nitrogen. The subsequent coating application on hot-dip galvanized steel samples at 180 °C was carried out by spray or wave application. During hot-dip galvanizing, the corresponding workpieces are generally treated in a dipping process. Therefore, the process to be developed focused on a wet-chemical process, which is developed as a spraying or dipping process. After cooling the coated planar samples to room temperature, various tests were carried out to evaluate the protective effect. For example, a quick test using Zinkcheck 1401 ZC [5], which is commonly used by powder coating companies for incoming goods inspection of galvanized components, was used.
Without protection or in the presence of a small amount of additional protection on galvanized surfaces, a time-dependent (in minutes) colour change of the dripped test solution from colourless to black can be observed (Fig. 7). 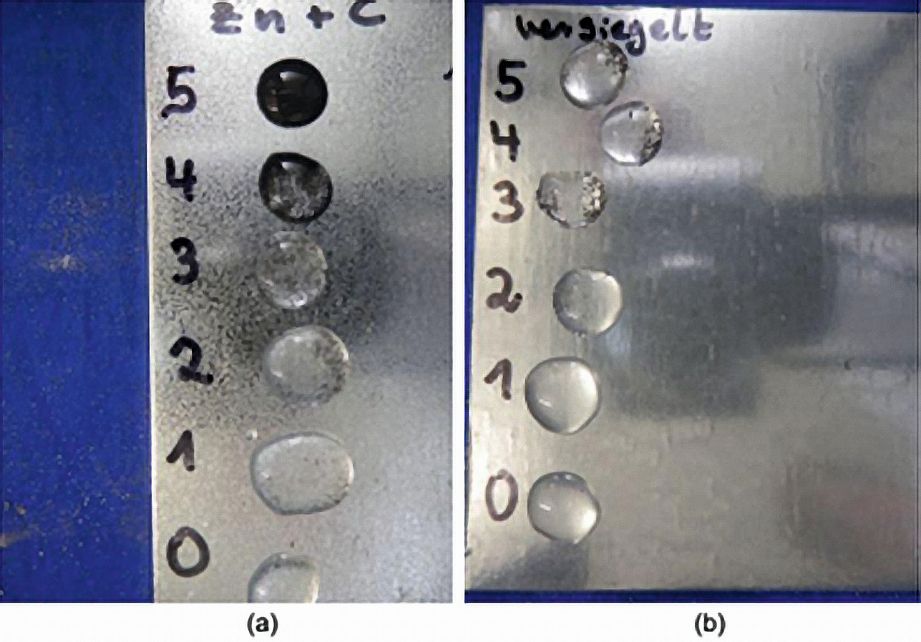 Fig. 7: Spot test zinc surfaces; left: time-dependent (in minutes) discoloration of the test solution on a chemically passivated zinc surface and right: absence of color change due to the presence of a sealant on the zinc surface
Fig. 7: Spot test zinc surfaces; left: time-dependent (in minutes) discoloration of the test solution on a chemically passivated zinc surface and right: absence of color change due to the presence of a sealant on the zinc surface
If, on the other hand, the zinc surface is sealed, the color change does not occur. As part of the research project, an increasingly delayed color reaction of the dripped test solution was observed as the proportion of polymer in the aqueous solution increased (Fig. 8).
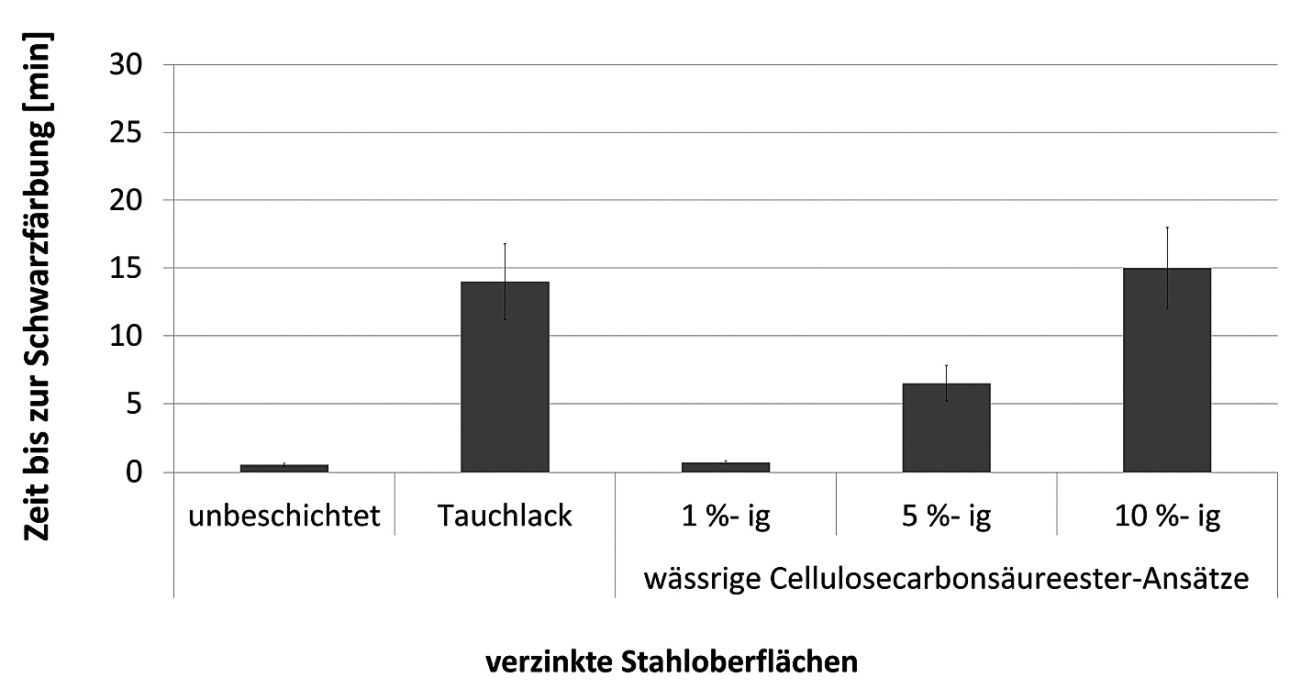 Fig. 8: Results with Zinkcheck 1401 ZC spot test of hot-dip galvanized steel samples
Fig. 8: Results with Zinkcheck 1401 ZC spot test of hot-dip galvanized steel samples
Using a 10 % aqueous polymer-based preparation, a result comparable to the dip coating commonly used on the market was achieved. In addition to the spot test investigations, a process-accompanying evaluation of the corrosion phenomena after artificial sample spraying was also carried out. At this point, a test setup from Otto Vision Technology GmbH (Fig. 9) was used for photographic documentation and gray value determination in a measuring range between 0 (black) and 255 (white).
During the measurements, the samples were irradiated with LEDs at a 45° angle from two sides. With increasing white rust infestation and an associated loss of gloss level, a clear increase in the measured gray values was observed on the basis of the tests (Fig. 10).
The gray value measurements also confirmed the correlation between polymer concentration and protective effect previously derived using the spot test (Fig. 11).
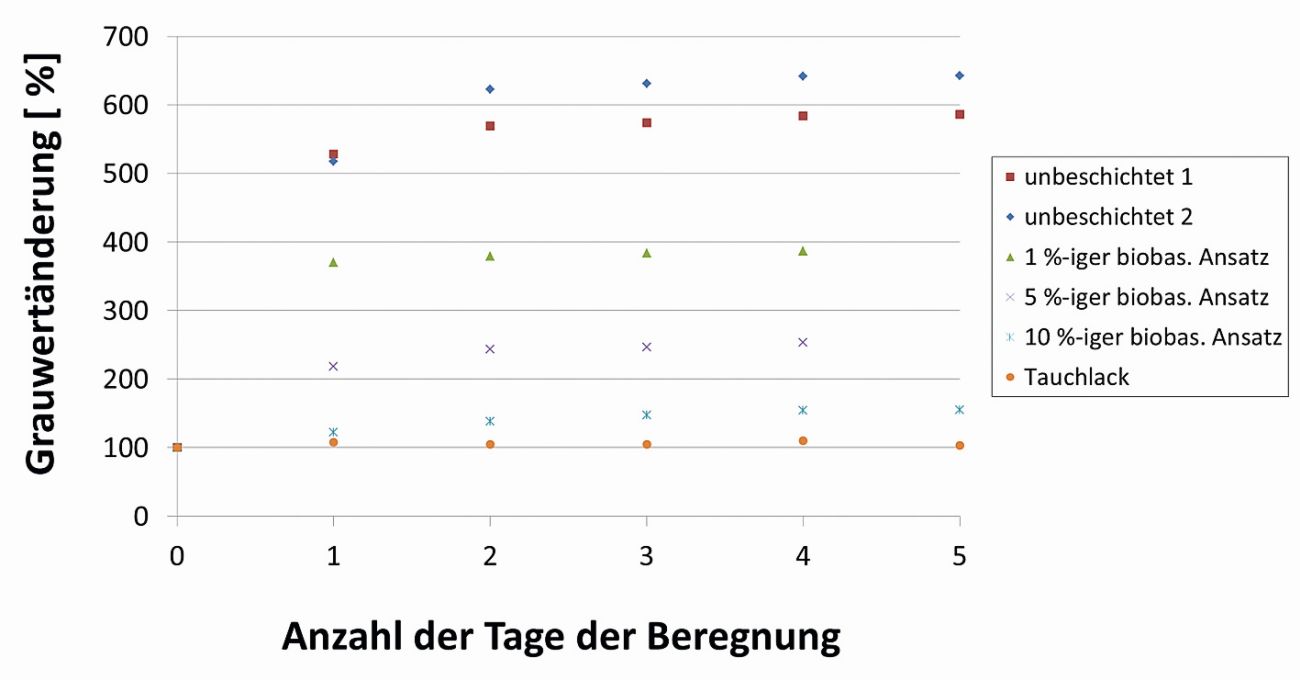 Fig. 11: Change in grey scale value (normalization to initial value) of hot-dip galvanized steel samples over an artificial irrigation period of 5 days
Fig. 11: Change in grey scale value (normalization to initial value) of hot-dip galvanized steel samples over an artificial irrigation period of 5 days
As described above, powder coatings are used for long-term corrosion protection of galvanized surfaces. To remove any run-off marks or spots caused by the galvanizing process, the surfaces are first smoothed and ground. This is followed by mechanical sweeping with an iron-free abrasive or a wet-chemical pre-treatment consisting of degreasing, pickling and chromium-free passivation [6, 7]. Any coatings applied for temporary protection after galvanizing are also removed during sweeping. Figure 12 shows a schematic of the wet chemical pre-treatment process at Bader Pulverbeschichtungs GmbH. In the active zone (180 seconds, 45-50 °C), Bader works with an acidic pickling agent.
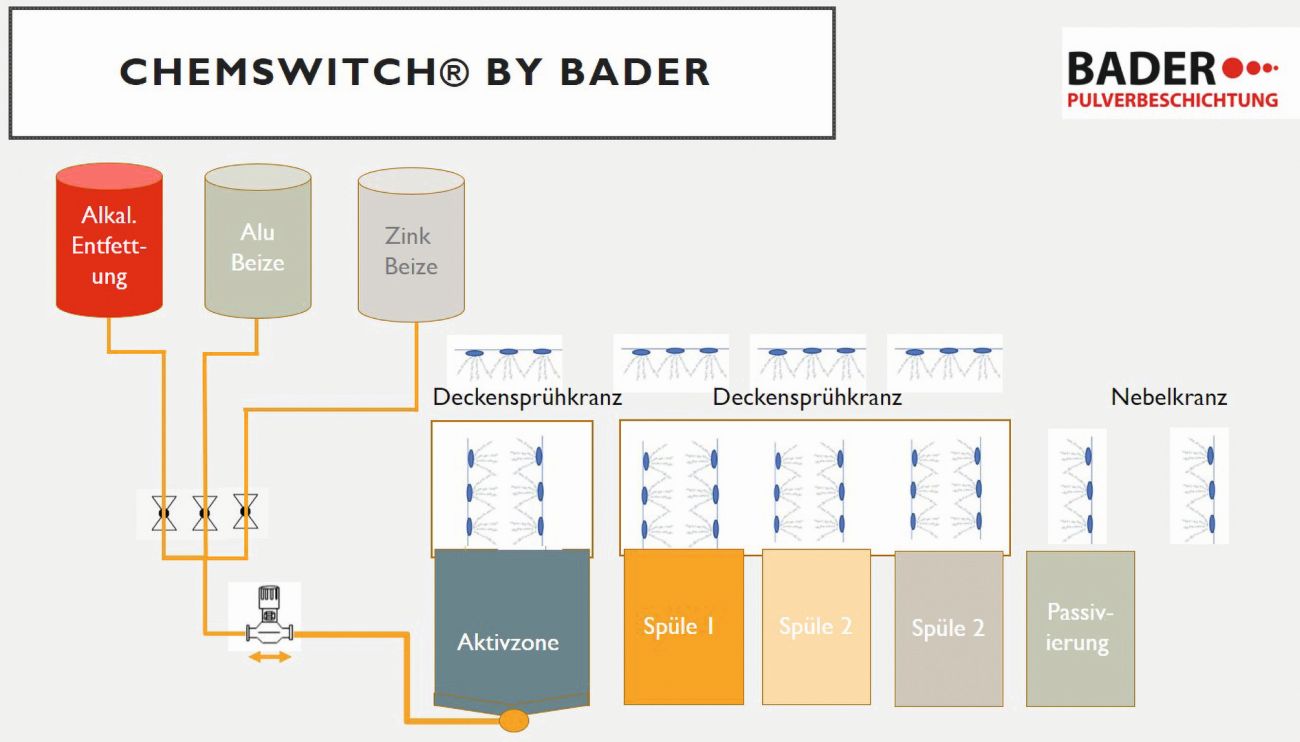 Fig. 12: Schematic of the wet chemical pre-treatment process at Bader Pulverbeschichtungs GmbH
Fig. 12: Schematic of the wet chemical pre-treatment process at Bader Pulverbeschichtungs GmbH
The project work has shown that the bio-based coating developed can be easily overcoated with conventional powder coatings and baked together. As a result, the process steps mentioned for wet-chemical or mechanical pre-treatment can be dispensed with. A boiling test according to DIN EN ISO 2409 [8] was used to evaluate the coated samples. The holding time was 120 seconds, which corresponds to stress group IV. Subsequent cross-cut examinations showed no flaking of the coating. In contrast to the dip coating commonly used as temporary protection, the quality of the powder-coated zinc surfaces after overcoating corresponded to the required quality (Fig. 13). This eliminates the need for the time-consuming removal of layers using mechanical and chemical processes prior to powder coating, which was previously necessary in the state of the art.
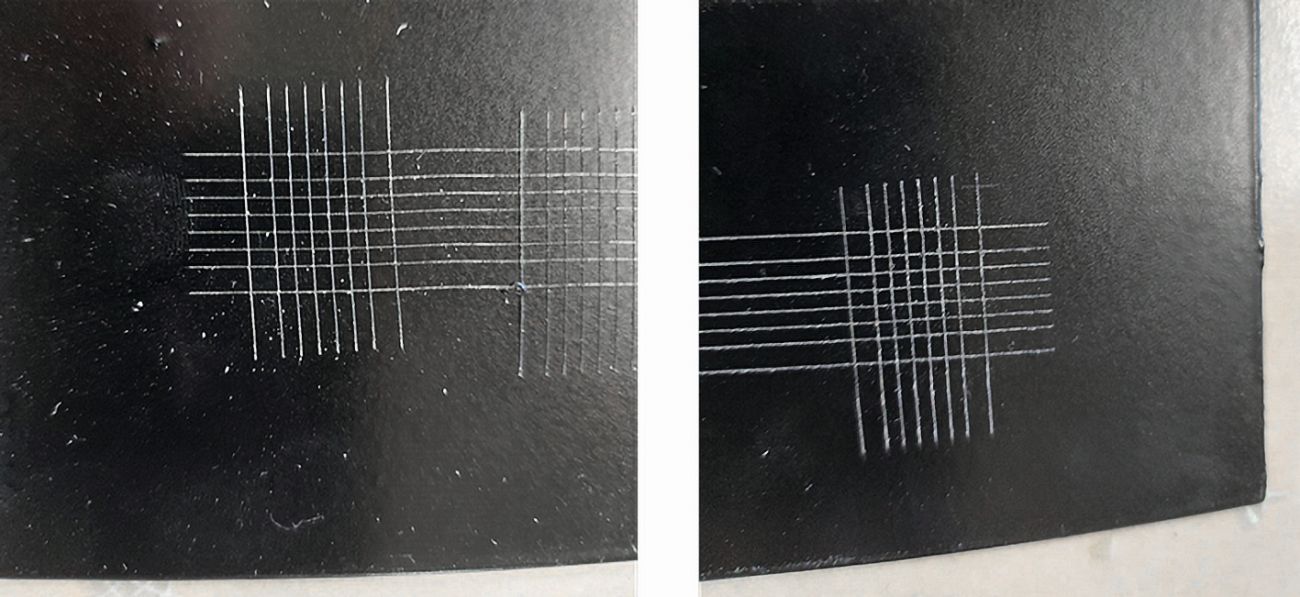 Fig. 13: Cross-section on biobased protected hot-dip galvanized steel surfaces subsequently coated with a powder coating; left sample: before and right sample: after 120 minutes of boiling test in accordance with DIN EN ISO 2409 (QIB stress group IV)
Fig. 13: Cross-section on biobased protected hot-dip galvanized steel surfaces subsequently coated with a powder coating; left sample: before and right sample: after 120 minutes of boiling test in accordance with DIN EN ISO 2409 (QIB stress group IV)
Conclusion
As part of a research project funded by the Federal Ministry for the Environment and Energy under the funding code 16KN071124, a bio-based temporary corrosion protection for zinc surfaces has been successfully developed [9]. A comparison with a state-of-the-art air-drying, water-based temporary protective coating (Fig. 8) once again illustrates the outstanding product properties. This development has laid a further important foundation for the use of renewable raw materials in the field of corrosion protection. The aim of future work is to further develop the protective effect so that corrosion phenomena that still occur in certain areas will be a thing of the past in future.
Literature
[1]El-Mahdy, A.; Nishikata, A.; Tsuru, T.; Corrosion Science: Electrochemical corrosion monitoring of galvanized steel under cyclic wet-dry conditions, 42, 2000, p. 183
[2]Beilstein J. Org. Chem. 2014, 10,1549-1556
[3]Meier, M.; Liebert, T.; Nagel, M. C. V.; Jordan, T.; Heft, A.; Grünler, B.; Heinze,T.; Macromolecular Rapid Communications: Pure, transparent-melting starch esters: Synthesis and Characterization, 2011, 32, pp. 1312-1318
[4]Becker, H.; Scherer, B.; Heft, A.; Grünler, B.; Restaurierung und Archäologie: Stärkeester - Ein nachhaltiges Korrosionsschutzsystem für archäologische Eisenobjekte, Verlag des Römisch-Germanischen Zentralmuseums, 2017, 10, pp. 95 - 108
[5]https://www.besserlackieren.de/technologien/metalllackierung/22_05_verzinkte-substratebeschichten
[6]Pietschmann, Industrial powder coating, Friedr. Vieweg & Sohn Verlag/GWV Fachverlage GmbH, Wiesbaden 2003
[7]https://www.poligrat.de/wp-content/uploads/2019/05/Beizen-Reinigen-Passivieren.pdf
[8]QIB - Quality specifications Rev. 14, 07/2017; DIN EN ISO 2409
[9]Keil, D.; Bader, M.; Journal für Oberflächentechnik: Overpaintable protection for zinc surfaces, 61, Special corrosion protection, 2021, p. 30 - 31