4 Corrosion diagnostics with agar gels
4.1 Use of gel electrolytes with indicators
Color indicators for the detection of certain ion species have been used for a long time to easily determine the presence, the reaction sites or even the quantities of the species. These simple electrographic detection methods can be used for corrosion diagnostics by specifically adjusting the corrosiveness of the electrolyte to detect critical defects or material inhomogeneities. The aqueous electrolyte with a certain corrosivity and an indicator is then applied to the surface for a certain period of time. Filter papers are often recommended as electrolyte carriers, although gel-like electrolyte carriers, e.g. agar-based, have clear advantages. The mobility of the complexed substances in the electrolyte carrier is responsible for the imaging quality of a color indication, which, as Figure 5 shows, depends largely on the pore size. Figure 6 shows three different electrolyte carriers with an aqueous electrolyte of 10 mmol/L NaCl and 1 mmol/L potassium hexacyanoferrate after 15 minutes on a steel sheet with an iron phosphate coating (0.6-1 g/m2).
![Abb. 5: Visualisierung der Diffusionshemmung unterschiedlich großer sphärischer Teilchen in Abhängigkeit der Porengröße gel-artiger Medien nach Ogston [27] und der Formel in Abbildung 4](/images/stories/Abo-2022-01/gt-2022-01-0050.jpg) Fig. 5: Visualization of the diffusion inhibition of spherical particles of different sizes as a function of the pore size of gel-like media according to Ogston [27] and the formula in Figure 4
Fig. 5: Visualization of the diffusion inhibition of spherical particles of different sizes as a function of the pore size of gel-like media according to Ogston [27] and the formula in Figure 4
The electrolyte film has access to the steel via pores and other defects in the phosphate layer, allowing iron ions to dissolve into the electrolyte. The iron ions migrate into the electrolyte carrier and are complexed, which leads to the formation of Berlin blue and indicates the presence, location, number and size of the pores. However, the quality of individual indications varies greatly. In a filter paper with a pore size of 15 µm (Fig. 6c), individual indicators blur into an almost flat blue coloration. With a smaller pore size, on the other hand, the colour complex is more strongly immobilized. At 1.5 µm (Fig. 6b), individual displays are already clearly recognizable and the blurring of the displays is less pronounced. However, the contrast and the display sharpness increase again significantly when using an agar gel with 3 % agar as gel former (Fig. 6a), which has a pore diameter of approx. 0.165 µm. In Figure 5, the inhibition of mass transport has already been shown as a model and the improved display sharpness can be explained by the stronger immobilization of the Berlin blue pigment.
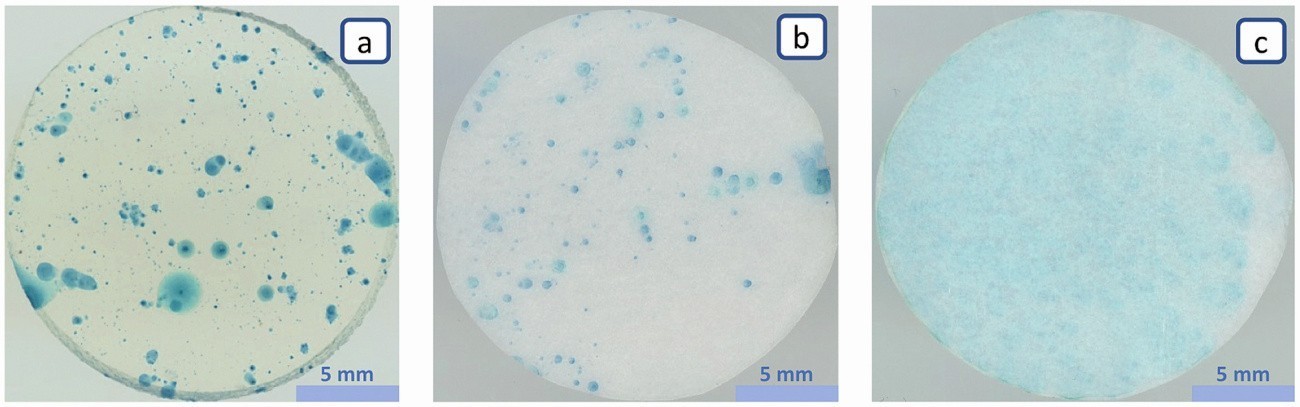 Fig. 6: Comparison of different electrolyte carriers with different pore sizes after 15 minutes on a steel with an iron phosphate coating, a) 3% agar gel with 0.165 µm pores, b) filter paper with 1.5 µm pores, c) filter paper with 15 µm pores
Fig. 6: Comparison of different electrolyte carriers with different pore sizes after 15 minutes on a steel with an iron phosphate coating, a) 3% agar gel with 0.165 µm pores, b) filter paper with 1.5 µm pores, c) filter paper with 15 µm pores
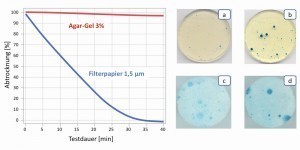 Fig. 7: Change in the test media over a period of 40 min due to drying and effect on the indication of weak points in the passive layer of the stainless steel X2CrNi18-10 with increased sulphur content, test time 15min, a) gel pad with 0.1 mol/L NaCl, b) gel pad with 0.3 mol/L NaCl, c) filter paper with 0.1 mol/L NaCl, d) filter paper with 0.3 mol/L NaCl (for potassium hexacyanoferrate content, see text) The use of gel-like electrolyte carriers has another decisive advantage over filter paper. The gels remain uniformly moist for longer and keep the electrolyte concentration constant for the duration of the test. Filter papers, on the other hand, absorb less electrolyte and release it much more quickly, which means that the electrolyte concentration changes rapidly during the test. The effects are dramatic, especially in applications where the corrosiveness caused by the ion concentration is particularly critical, e.g. with passively present stainless steels in contact with a chloride-containing electrolyte. Figure 7 shows the comparison between gel electrolyte and filter paper with 0.1 and 0.3 mol/L NaCl and 1 and 3 mmol/L potassium hexacyanoferrate respectively. As the filter paper dries, the electrolyte loses almost 40 % of its water in 10 min and the NaCl concentration increases accordingly. It can be seen that, in contrast to the constantly moist gel, a differentiated test of the pitting corrosion resistance with filter papers is not possible, as the initially low chloride content also results in severe pitting corrosion after a short time due to the increased concentration.
Fig. 7: Change in the test media over a period of 40 min due to drying and effect on the indication of weak points in the passive layer of the stainless steel X2CrNi18-10 with increased sulphur content, test time 15min, a) gel pad with 0.1 mol/L NaCl, b) gel pad with 0.3 mol/L NaCl, c) filter paper with 0.1 mol/L NaCl, d) filter paper with 0.3 mol/L NaCl (for potassium hexacyanoferrate content, see text) The use of gel-like electrolyte carriers has another decisive advantage over filter paper. The gels remain uniformly moist for longer and keep the electrolyte concentration constant for the duration of the test. Filter papers, on the other hand, absorb less electrolyte and release it much more quickly, which means that the electrolyte concentration changes rapidly during the test. The effects are dramatic, especially in applications where the corrosiveness caused by the ion concentration is particularly critical, e.g. with passively present stainless steels in contact with a chloride-containing electrolyte. Figure 7 shows the comparison between gel electrolyte and filter paper with 0.1 and 0.3 mol/L NaCl and 1 and 3 mmol/L potassium hexacyanoferrate respectively. As the filter paper dries, the electrolyte loses almost 40 % of its water in 10 min and the NaCl concentration increases accordingly. It can be seen that, in contrast to the constantly moist gel, a differentiated test of the pitting corrosion resistance with filter papers is not possible, as the initially low chloride content also results in severe pitting corrosion after a short time due to the increased concentration.
Another advantage is the significantly better contrast and the sharp image of the displays. This makes it possible to subsequently subject the images to a semi-automatic image analysis, which is based, for example, on color space separation and the detection of round objects. The use of filter papers does not provide any useful input data for image analysis. However, data for further digital processing can be generated immediately from the images of the gel pads, as Figure 8 illustrates. When detecting local weak points, such as pores in phosphating or pitting corrosion on passive surfaces, the number of indications and their percentage of area can be determined. Classification according to the size of the indications is also possible, allowing the types of defect to be differentiated. A large display is associated with a longer and stronger metal resolution and therefore larger defects. In the case of smaller indications, the metal dissolution at the location is significantly lower, i.e. the defects are either smaller, occur later, grow more slowly or repassivate. The released iron ions spread in all directions into the gel, where they react with the indicator. This results in circular indications. Longitudinal defects or damage to the layer can lead to bead-like indications, which are often recognized as one large indication in image processing.
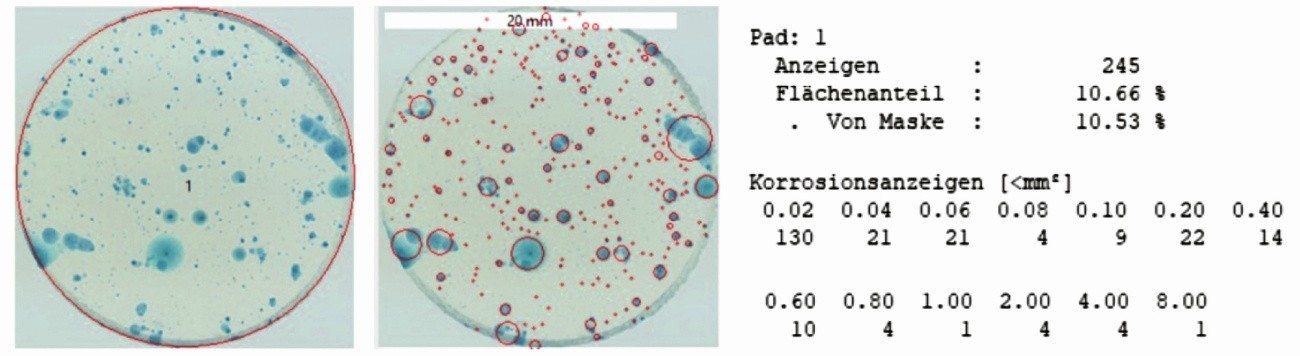 Fig. 8: Semi-automatic digital image analysis based on color space separation and object recognition for the gel pad in Figure 6a; the number and area percentage of the indicators are determined as well as a classification of the indicators according to their size (classification)
Fig. 8: Semi-automatic digital image analysis based on color space separation and object recognition for the gel pad in Figure 6a; the number and area percentage of the indicators are determined as well as a classification of the indicators according to their size (classification)
4.2 Use of gel electrolytes for electrochemical measurements
The use of indicators in gel electrolytes to display metal cations from corrosion reactions has the disadvantage that a relatively high metal dissolution is required. In addition, the limit above which, for example, a sufficiently dense protective layer is present or a passive layer is resistant must be specifically set via the corrosiveness of the gel and thus determined in advance. However, it is often desirable to analyze a surface almost non-destructively or to obtain a quantitative statement on the degree of resistance or the current (often very low) corrosion rate. The application of electrochemical measurement methods, which are used purely analytically for corrosion diagnostics, is predestined for these questions. The surface to be tested should be changed as little as possible by the measurement itself. This is achieved on the one hand by the correct choice of measurement method and measurement parameters. However, the gel electrolyte and its properties also have a favorable effect. Not only is the sensor technology simpler, as liquid electrolytes are no longer used, but the corrosiveness can be reduced even further. Below are some examples where gel electrolytes have already been successfully used with electrochemical measurement methods for corrosion diagnostics.
4.2.1 Simplification of sensor technology for laboratory and field applications
 Fig. 9: Simplification of the measurement set-up for electrochemical measurements with gel electrolytes, left: freely supported gel contacted with counter and reference electrode, right: prototype of a sensor for point-of-care applications with planar electrode coupling and defined contact pressure The gel electrolyte has the advantage that the coupling to the sample or the measurement object can be realized more easily, as no sealing cell is required to prevent the electrolyte from leaking. For so-called point-of-care applications, i.e. directly on real components in production or on structures outdoors, measurements can be carried out on site with such a sensor, even on vertical structures or overhead. Figure 9 shows the simplified measurement setup as it is often realized in the laboratory (left) and a sensor for field applications (right). The reference and counter electrodes are coupled to one side of the gel, the sample to the other. The contact pressure is controlled or realized by a spring element in the sensor so that the gel has uniform contact with the test surface but is not crushed. If the test object is magnetic, such as galvanized steel, the sensor can be attached to the test surface using magnets.
Fig. 9: Simplification of the measurement set-up for electrochemical measurements with gel electrolytes, left: freely supported gel contacted with counter and reference electrode, right: prototype of a sensor for point-of-care applications with planar electrode coupling and defined contact pressure The gel electrolyte has the advantage that the coupling to the sample or the measurement object can be realized more easily, as no sealing cell is required to prevent the electrolyte from leaking. For so-called point-of-care applications, i.e. directly on real components in production or on structures outdoors, measurements can be carried out on site with such a sensor, even on vertical structures or overhead. Figure 9 shows the simplified measurement setup as it is often realized in the laboratory (left) and a sensor for field applications (right). The reference and counter electrodes are coupled to one side of the gel, the sample to the other. The contact pressure is controlled or realized by a spring element in the sensor so that the gel has uniform contact with the test surface but is not crushed. If the test object is magnetic, such as galvanized steel, the sensor can be attached to the test surface using magnets.
Simplifying the sensor technology also raises the question of which electrochemical measurement methods can or should be used. There is no general answer to this question, as it depends on the particular problem and specific application. Ultimately, electrochemistry is used in corrosion research to analyze a corrosion system. This can either be done in a minimally invasive way, i.e. a determination of characteristic values can be carried out with the aim of not significantly changing the system. However, it is also possible to bring the system under investigation into a critical range by means of polarization and then check its reaction to this. One example would be the targeted induction of pitting corrosion in stainless steels at a critical potential. Both strategies can also be implemented with gel electrolytes, however, the induction of corrosion is essentially subject to the characteristics of the gel electrolyte, i.e. the change in mass transport through the gel has a decisive influence on the course of corrosion and the appearance of the corrosion phenomena.
For applications aimed at a minimally invasive description of a corrosion system, the inhibition of mass transport processes in gel electrolytes is an advantage. As the corrosion reactions are initially inhibited, the condition of the examined surface remains constant and analyzable for longer. This makes it possible to use measurement methods that have a longer analysis time window, such as impedance spectroscopy (several minutes, depending on the lowest frequency). In order to change the surface as little as possible during the electrochemical measurement, the selection of the measurement parameters is important. To determine an instantaneous corrosion current, one usually manipulates current or potential (abruptly, linear-dynamically or as sinusoidal oscillations of a certain frequency) and measures the respective counterpart (potential or current). As a rule of thumb, it can be said that the respective excitation amplitude should never be higher than the expected corrosion current so that the corrosion system is not affected. Once a characteristic value in the form of a current density or an area-related polarization resistance has been determined, it can be used for the evaluation of the system under investigation. This makes it possible to assess the presence and protective effect of coatings or to predict the further course of corrosion. Specific applications are presented in the following examples.
4.2.2 Detection of volatile corrosion inhibitors (VCI) on steel
Vapor phase or volatile corrosion inhibitors (VCI) are often used as part of a temporary corrosion protection strategy for the protection of metal parts in packaging and storage. It is not yet possible to test the presence and effectiveness of VCIs directly on products or in packaging. The effectiveness is therefore tested by storing metal samples in sealed containers containing VCI substances. The protective capacity is evaluated after the tests by comparing the appearance of the samples with predefined corrosion diagrams. The application of electrochemical methods has so far failed due to the fact that the few layers of molecules on the surface could not withstand the test conditions when liquid electrolytes were used and therefore no usable results were obtained. This is not surprising, as VCIs are designed to work in an atmosphere and not as an inhibitor for an aqueous medium. However, if gel electrolytes are used instead of aqueous electrolytes, the degradation of the VCI substances by the test electrolyte can be delayed to such an extent that electrochemical measurements are possible, on the basis of which the presence and effect of the VCI can be verified. In addition, short-term and minimally invasive electrochemical excitation is necessary to determine the characteristic values so that the phase boundary is changed as little as possible by the measurement, but a usable measurement signal is still obtained. Figure 10 shows a pulse measurement with a low current excitation (upper part) of ±1 µA with a gel pad of 6 mm diameter (corresponds to approx. ±3.5 µA/cm2 total amplitude). The middle section shows the course of the corrosion potential, on which the polarization is superimposed by the current pulses. The position and course of the potential are hardly changed by the alternating cathodic/anodic excitation with low amplitude, i.e. the influence on the corrosion system is minimal. The polarization component (lower part) can be extracted from the potential curve and thus the overall polarization can be evaluated.
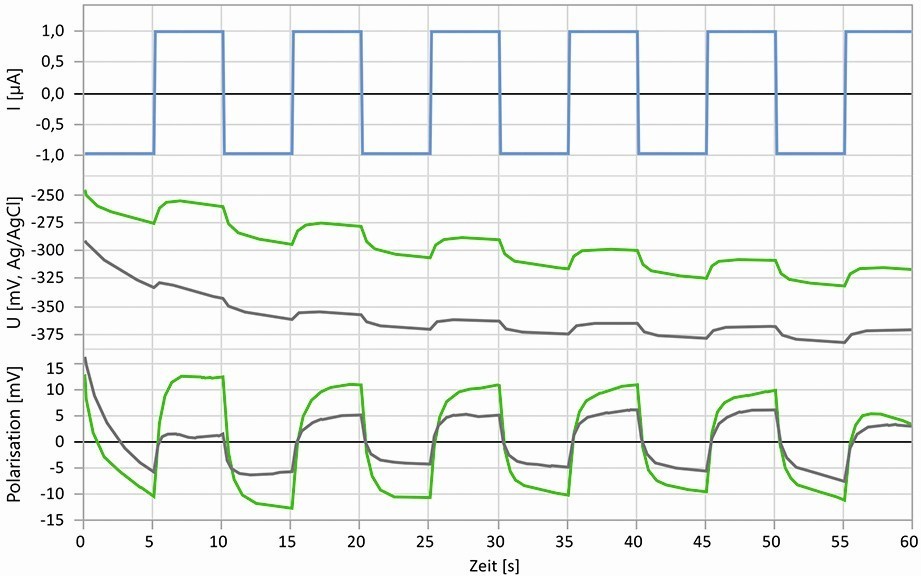 Fig. 10: Minimally invasive galvanostatic pulse measurements over 60 seconds on untreated (gray) steel and steel aged in a VCI atmosphere (green), top: Excitation with pulse current, center: potential curves, bottom: Polarization curves
Fig. 10: Minimally invasive galvanostatic pulse measurements over 60 seconds on untreated (gray) steel and steel aged in a VCI atmosphere (green), top: Excitation with pulse current, center: potential curves, bottom: Polarization curves
The gray curve represents the measurement on an untreated steel surface, the green curve was recorded on a steel surface that had been stored in a VCI atmosphere for 24 hours at the time of the measurement. The potential is significantly more positive for the VCI-treated steel, which already indicates an inhibition of metal dissolution. With the selected current excitation, the total polarization results in a polarization resistance of approx. 3860 Ωcm2 compared to approx. 1715 Ωcm2 for the condition without VCI and shows the inhibition of corrosion reactions and thus a certain protective effect. In this way, it can be quickly and reliably proven that the surface is covered with VCI and that protection is present. By using the sensors shown in Figure 9, such measurements can also be easily carried out on products or in packaging.
This example illustrates that the use of gel-like electrolytes and minimally invasive electrochemical methods enables metrological access to this type of "invisible" surface modification. Also conceivable are, for example, the testing of pre- and post-treatments of metal surfaces, in particular systems with very low layer weights, which already achieve an effect with mono-molecular coating of the surface. Corrosion diagnostics with gel-type electrolytes can also be used for applications in which thin films are deposited or passive or conversion coatings are part of a corrosion protection strategy.
- to be continued -
Literature
[1] Laque, F.L.; May, T.P.; Uhlig, H.H.: Corrosion in Action, International Nickel Company Canada, 1955
[2] Isaacs, H.S.; Adzic, G.; Jeffcoate, C.S.: Visualizing Corrosion, Corrosion 56 (2000) 10, 971-978, https://doi.org/10.5006/1.3294386
[3] DIN EN ISO 10309:2016-08: Metallic coatings - Test method for the determination of porosity - Ferroxyl test (ISO 10309:1994), German version EN ISO 10309:2016
[4] Petersen, P.; Emnéus, H.: The Ferroxyle Test as a General Test of the Corrosiveness of Surgical Appliances Made from Stainless Steel or Co-Based Alloys of Stellite- Type, Mainly Vitallium and Neutrilium, Acta Orthopaedica Scandinavica, 29, 1-4, 1959, 331-340, https://doi.org/10.3109/17453675908988808
[5] Lehmann, J.; Burkert, A.; Müller, T.; Bohlmann, T.; Burkert, A.: Final report on the IGF project 17136 N/1 Detection of corrosion-sensitive surfaces of stainless steels by the processors, available on researchgate.net
[6] Patent specification DE 10 2010 037 775 B4: Condition for the detection of corrosion-sensitive metal surfaces and method for the detection of corrosion-sensitive metal surfaces, patent granted on 8.5.2014
[7] Rosemann, P.; Kauss, N.; Heyn, A.: KorroPad-Prüfung - Anwen[1] dungen aus Industrie und Forschung, 3-Länder-Korrosionstagung - Korrosion ist kein Zufall - Neue Messmethoden, Analytik und Simulation, May 2019, Frankfurt a. Main, available on researchgate.net
[8] Rosemann, P.; Müller, T.; Babutzka, M.; Heyn, A.: Influence of microstructure and surface treatment on the corrosion resistance of martensitic stainless steels 1.4116, 1.4034, and 1.4021, Materials and Corrosion, 66 (2015), 45-53, https://doi.org/10.1002/maco.201307276
[9] Reinemann, S.; Babutzka, M.; Rosemann, P.; Lehmann, J.; Burkert, A.: Influence of grinding parameters on the corrosion behavior of austenitic stainless steel, Materials and Corrosion, 70 (2019), 1776-1787, https://doi.org/10.1002/maco.201910874
[10] Kauss, N.; Heyn, A.; Halle, T.; Rosemann, P.: Detection of sensitization on aged lean duplex stainless steel with different electrochemical methods, Electrochimica Acta 317 (2019) 17-24, https://doi.org/10.1016/j.electacta.2019.05.081
[11] Newton, C.J.; Sykes, J.M.: A galvanostatic pulse technique for investigation of steel corrosion in concrete, Corrosion Science, 28, (1988), No. 11, 1051-1074, https://doi.org/10.1016/0010-938X(88)90101-1
[12] Spark, A.J.; Cole, I.; Law, D.; Ward, L.: The effect of peptide based nutrients on the corrosion of carbon steel in an agar based system, Corrosion Science 110, 2016, 174-181, https://doi.org/10.1021/acs.est.7b00437
[13] Spark, A. J.; Cole, I.; Law, D.; Marney, D. and Ward, L.: Investigation of agar as a soil analogue for corrosion studies. Materials and Corrosion 67 (2016), pp 7-12, https://doi.org/10.1002/maco.201508312
[14] Spark, A.J.; Law, D.W.; Ward, L.P.; Cole, I.S.; Best, A.S.: Effect of Pseudomonas fluorescens on Buried Steel Pipeline Corrosion, Environmental Science & Technology, 51 (15), 2017, 8501-8509, https://doi.org/10.1021/acs.est.7b00437
[15] Vanbrabant, J.; van de Velde, N.: Industrial application of an electrochemical corrosion test using a gel matrix as simulation for atmospheric and solid media, Proceedings: European General Galvanizers Association Intergalva Berlin, Vol. 19, June 7th, 2000, 29/1 to 29/13
[16] Shao, X.M.; Feldman, J.L.: Micro-agar salt bridge in patch-clamp electrode holder stabilizes electrode potentials, Journal of Neuroscience Methods 159, 2007, 108-115, https://doi.org/10.1016/j.jneumeth.2006.07.001
[17] Monrrabal, G.; Guzmán, S.; Hamilton, I.E.; Bautista, A.F.; Velasco, F.: Design of gel electrolytes for electrochemical studies on metal surfaces with complex geometry, Electrochimica Acta, 220 (2016), 20-28, http://dx.doi.org/10.1016/j.electacta.2016.10.081
[18] Monrrabal, G.; Ramírez-Barat, B.; Bautista, A.; Velasco, F.; Cano, E.: Non-Destructive Electrochemical Testing for Stainless-Steel Components with Complex Geometry Using Innovative Gel Electrolytes. Metals,8 (2018), 500, https://doi.org/10.3390/met8070500
[19] Monrrabal, G.; Huet, F.; Bautista, A.: Electrochemical noise measurements on stainless steel using a gelled electrolyte, Corrosion Science, Volume 148, March 2019, Pages 48-56, https://doi.org/10.1016/j.corsci.2018.12.004
[20] Monrrabal, G.; Bautista, A.; Valesco F.: Use of Innovative Gel Electrolytes for Electrochemical Corrosion Measurements on Carbon and Galvanized Steel Surfaces, CORROSION, 75 (2019), 12, 1502-1512, https://doi.org/10.5006/3309
[21] Cano, E.; Crespo, A.; Lafuente, D.; Ramirez Barat, B.: A novel gel polymer electrolyte cell for in-situ application of corrosion electrochemical techniques, Electrochemistry Communications, 41 (2014), 16-19, https://doi.org/10.1016/j.elecom.2014.01.016
[22] Di Turo, F.; De Vito, C.; Coletti, F.; Mazzei, F.; Antiochia, R.; Favero, G.: A multi-analytical approach for the validation of a jellified electrolyte: Application to the study of ancient bronze patina, Microchemical Journal, Vol. 134, 2017, 154-163, https://doi.org/10.1016/j.microc.2017.05.015
[23] Babutzka, M.; Burkert, A.; Heyn, A.: Korrosionsuntersuchungen mit gelartigen Elektrolyten zur Beschreibung der Korrosionsschutzwirkung von Zinküberzügen, 16th Summer Course Materials and Joining: Magdeburg, September 8-9, 2017, 119-128, http://dx.doi.org/10.25673/5002
[24] Babutzka, M.; Heyn, A.: Dynamic tafel factor adaption for the evaluation of instantaneous corrosion rates on zinc by using gel-type electrolytes, IOP Conf. Ser.: Mater. Sci. Eng. 181, 2017, 012021, https://doi.org/10.1088/1757-899X/181/1/012021
[25] Labille, J.; Fatin-Rouge, N.; Buffle, J.: Local and Average Diffusion of Nanosolutes in Agarose Gel: The Effect of the Gel/Solution Interface Structure, Langmuir, 23 (2007), 2083-2090, https://doi.org/10.1021/la0611155
[26] Vaucher, S.; Li, M.; Mann, S.: Synthesis of Prussian Blue Nanoparticles and Nanocrystal Superlattices in Reverse Microemulsions, Angew. Chem. Int. ed, 39, 1793-1796 http://www.doi.org/10.1002/(SICI)1521-3773(20000515)39:10<1793::AID-ANIE1793>3.0.CO;2-Y
[27] Ogston, A.G.; Preston, B.N.; Wells, J.D.: On the transport of compact particles through solutions of chain polymers, Proc. R. Soc. London, A. 1973333 (1973), 297-316, https://doi.org/10.1098/rspa.1973.0064
[28] Somma, M.; Querci M.: The Analysis of Food Samples for the Presence of Genetically Modified Organisms, Session 5: Agarose Gel Electrophoresis, 62, https://doi.org/10.2760/5277
[29] Draft standard for a test method for the determination of surface layer resistances on zinc coatings using gel-like electrolytes - GELELEK, research project in the BMWi WIPANO research initiative, Project Management Jülich, Berlin
[30] Killik, A.: Influencing factors on the corrosion of differently galvanized steel test specimens in short-term corrosion tests and in the field, Dissertation, Otto von Guericke University Magdeburg, 2016
[31] J.R. Scully: Polarization Resistance Method for Determination of Instantaneous Corrosion Rates, Corrosion 56 (2000) No. 2, 199-218, NACE International, https://doi.org/10.5006/1.3280536
[32] ASTM G3-14: Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing, Reapproved 2019
[33] DIN EN ISO 9223:2012: Corrosion of metals and alloys - Corrosivity of atmospheres - Classification, determination and estimation
[34] Heyn A.: Bewertung der Korrosivität von Atmosphären anhand von Wetterdaten, 16th Summer Course Materials and Joining: Magdeburg, September 8 and 9, 2017, 129-138, http://dx.doi.org/10.25673/5002


