Highly pigmented zinc dust primers ("zinc rich primers", ZRP) can provide long-term protection against corrosion for steel structures, even under highly corrosive conditions. Here, the zinc pigments act as sacrificial anodes that provide cathodic corrosion protection when in electrical contact with the substrate.
However, the resulting zinc oxidation products are considered hazardous to water. Since high zinc pigment concentrations also result in a low binder content and optimum surface preparation cannot always be guaranteed under difficult application conditions, zinc pigment primers with a reduced zinc content could be advantageous if formulations with a corrosion protection effect comparable to that of ZRP could be identified by modifying the pigment composition. In the following, various approaches to such formulations are outlined and methods are presented with which the potential of such formulations could be assessed.
If the zinc content in zinc pigment primers is to be reduced while maintaining the protective effect, the following options are basically available for modifying the formulation if these are only to affect the zinc pigment itself:
- Varying the particle size: typically, zinc pigments with a diameter of around 3.5 µm are used; increasing the diameter (while keeping the zinc content constant) causes a reduction in the active surface area.
- Variation of the particle shape: In addition to the spherical zinc pigments that have been commercially available to date, platelet-shaped pigments in particular could be used, which offer the prospect of an increase in the active surface area and an additional barrier effect at the same content; non-leafing products should always be used, as no concentration near the interface is initially desired.
- Variation of the surface: If the surface of the zinc pigment is organically coated by a suitable deposition, this can result in a reduction of the activity of the zinc pigment - possibly combined with a prolonged effectiveness.
- Composition: This can be achieved either by zinc alloy pigments or by metallic deposits on zinc pigments; both should influence the initial electrochemical potential of the pigment and can also mean additional ecological advantages, for example when magnesium is used.
- Addition of "inert conductivity" pigments: Although these do not provide any cathodic protection in themselves, they cause a permanent increase in electrical conductivity and thus influence the activity (duration) of the zinc pigment.
As already mentioned above, the corrosion protection provided by zinc primers consists of cathodic corrosion protection [1], which is based on the low electrochemical potential of zinc, and the barrier effect of the voluminous zinc oxidation products formed as a result of exposure to corrosive agents, which close the pores in the binder ("cementation") [2]. Figure 1 shows these two protection mechanisms (pcp: "phase of cathodic protection", bp: "barrier phase").
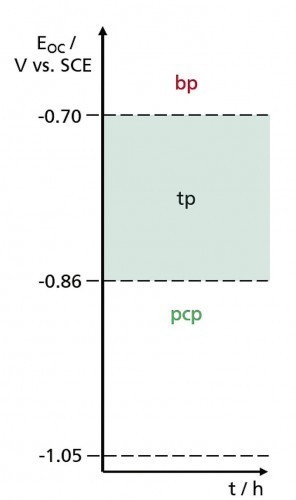 Fig. 1: Schematic representation of the protective effect phases of zinc primers
Fig. 1: Schematic representation of the protective effect phases of zinc primers
As long as there is contact between the steel surface to be protected and the zinc pigments, the latter are preferentially oxidized up to the electrochemical potential of the steel (approx. -0.86 V vs. SCE) when exposed to corrosive agents.
preferentially oxidized. Above this potential, cathodic protection of the steel substrate can only be partially guaranteed. This transition phase (tp) ends at -0.70 V vs. SCE. At higher potentials, the primer can only protect the steel surface by acting as a barrier due to the cementation effect.
To estimate the initial electrochemical effect of the five measures mentioned above, the (mostly zinc-containing) pigments listed in Table 1 were homogeneously dispersed in a 30 wt.% concentration in a slightly alkaline binder (Wörwag Mischlack colorless, M1471, pH 8.5) and applied to untreated, cleaned and corrosion-free steel sheets.
| Pigment |
Type, D50 |
Ecor / V vs. SCE |
icor / A/cm2 |
| CONMET Superfine 620 | Standard zinc dust, ø 3.5 µm | -0,932 |
3,23∙10-6 |
| CONMET Special 615 | Zinc dust, ø 5 µm | -0,907 |
~ 1∙10-11 |
|
CONMET Superfine 620 + |
Standard zinc dust, ø 3.5 µm | -0,805 |
~ 1∙10-11 |
| ECKART Standard Zinc flake GTT | Zn flake, length 13.7 µm | -0,907 |
2,37∙10-7 |
| ECKART Stapa 4 ZnSn30 |
Zn/Sn alloy pigment, |
-0,826 |
1,33∙ 10-6 |
| ECKART Stapa 15 ZnMg26 |
Zn/Mg alloy pigment, |
-1,245 |
2,87∙10-7 |
| THE SIXTH ELEMENT Graphene SE1132 |
Graphene, < 3 wt.% O, |
-0,229 |
7,29∙10-7 |
|
Mg2Pi↓Zn |
Zn precipitate / Mg pigment, |
-0,583 |
8,45∙10-6 |
After conditioning, the 15 µm thick films were anodically and cathodically polarized linearly on two different surfaces based on the open-circuit potential of this sample under the influence of a 3% NaCl solution. From this, the corrosion potentials and current densities (Ecor andicor) given in Table 1 were obtained. The latter show that in the case of (i) the large spherical zinc pigments (CONMET Special 615, ø(D50) 5 µm) and in the case of (ii) the standard zinc dust equipped with a surface treatment, the specified zinc pigment concentration is not sufficient to achieve a sufficiently low penetration resistance. This can be explained by the fact that the charge transfer through the zinc pigments is impeded due to the reduced total number in case (i) or the partial surface passivation in case (ii). In case (ii), the significantly increased corrosion potential compared to the non-surface-treated standard zinc dust is also noticeable. Apparently, the surface treatment significantly "refines" the zinc surface. As expected, this also occurs when the ZnSn alloy pigment is used, whereas the ZnMg pigment consequently has a potential significantly below that of the zinc dust. The purely graphene-based formulation - since graphene does not provide cathodic corrosion protection - has the highest potential of all the potentials measured here. The potential of the Mg pigment on which Zn was deposited is surprisingly high. As was shown in subsequent investigations, partial oxidation already occurred here during deposition; the pigment is also unstable in the presence of water (even with pH regulation) and therefore cannot be used to formulate corrosion protection primers.
formulation of corrosion protection primers.
In the following, some new methods are presented with which the mechanism of action and corrosion protection properties of potentially market-relevant zinc primers can be determined in a relatively short time. The time saving is achieved by using thermocyclic (electrolytic) exposure (TEB), in which the sample is coated with an aqueous solution and subjected to a short-period - typically sinusoidal - temperature modulation [3, 4]. The latter is based on the fact that natural outdoor weathering usually takes place in such thermal cycles. A time-lapse of such cycles increases the probability of non-damage-free relaxation of the thermocyclically induced internal stresses that build up. The latter are caused by the post-crosslinking or interlocking of polymer strands of the binder. Since the temperature fluctuations cause the coating to either permanently expand or contract - and since the substrate generally has a coefficient of thermal linear expansion that differs from that of the coating - the aforementioned polymer strand entanglements and post-crosslinking result in the accelerated formation of cohesive and adhesive weak points and the formation of pores or cracks in the polymer. In the presence of an electrolyte overlaying the coating, this contributes to its rapid penetration onto the substrate and, as a result of the expansion-contraction cycles, also to its forced exchange and thus to increased corrosive stress.
It has been shown that continued moderate thermocycling (temperature window: 10 °C ... 40 °C, period duration: 1 h, sinusoidal) under the influence of a 3% NaCl solution in combination with regular detection of the open-circuit potential (EOC) of the zinc primers to be tested allows a good prediction of the outdoor weathering behavior of these systems when exposed to seawater. Specifically, steel test panels (Sa 2.5) coated with primer formulations that varied with the zinc pigments were exposed to the spray water zone in Helgoland for one year. Duplicates of these samples were characterized with regard to the EOC curve during a 420-hour exposure as described above. For each formulation, the parameter tbp was extracted from the EOC curve, which corresponds to the time of the potential transition from the transition phase (tp) to the barrier phase ( see Fig. 2).
If the zinc primer test panels assessed after one year of exposure in Helgoland (spray water zone) are arranged in the sequence of descending tbp values, the result shown in Figure 3 is that tbp can be used as an indicator for the maritime outdoor exposure of zinc primers. It must be added that this correlation is only valid if the corrosion protection of the primer is essentially based on the zinc pigments. In the case of primers with a significantly reduced zinc content, tbp loses its significance. The same applies if the formulations contain other metals that are electrochemically active in relation to the substrate in addition to zinc, as these influence EOC and therefore also tbp.
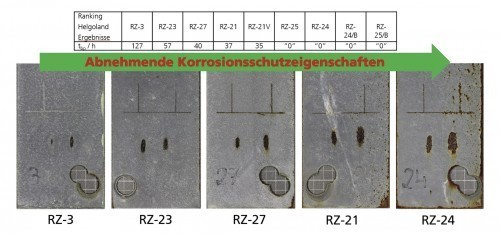 Fig. 3: Comparison of the tbp values determined for zinc primers with the exposure results obtained after one year of Helgoland exposure
Fig. 3: Comparison of the tbp values determined for zinc primers with the exposure results obtained after one year of Helgoland exposure
Artificial thermocyclic-hydrolytic exposure can also be used to specifically study the kinetics and effects of water penetration into the coating. Since water has a dielectric constant of εr ≈ 80, whereas this value does not exceed 10 in the case of organic coatings, and since the coating capacity C is proportional to εr, a steady increase in C reliably indicates water uptake. Although an exact determination of the water content of coatings via C is not trivial, the C curves determined during a thermocyclic-hydrolytic exposure can provide information about the relative water content changes caused by this in the stressed coating samples. The benefits of such investigations will be demonstrated below for a series of predominantly zinc-containing corrosion protection primers.
For this purpose, test plates of the systems described below were covered with hollow cylinders (ø 25 mm), filled with 6 ml of a non-corrosive electrolyte solution (0.025 M KNO3) and subjected to short-period thermocycling (10 °C ... 40 °C, period duration: 30 min, sinusoidal T-modulation of the electrolyte solution) and continuously detected by means of an impedance analyzer with regard to the high-frequency impedance modulus |Z|HF. If the phase shift is close to 90°, the coating capacitanceCHF can be approximated from this:CHF ≈ (2πf|Z|HF)-1. Here, f corresponds to the frequency of the excitation signal applied for detection by the impedance analyzer and is typically around 100 kHz.
Figure 4 shows the CHF values detected over a thermocycling period of 480 min for a total of seven corrosion protection primers. The three ZS systems contain 50 % zinc by volume and the two Zx systems contain 25 % zinc by volume. The two Zad systems, on the other hand, are corrosion protection primers without elemental zinc. The diagram on the right - which does not include the ZS systems - is a scale enlargement of the diagram on the left.
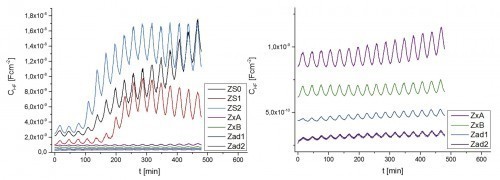 Fig. 4: t/CHF diagrams of seven corrosion protection primers, recorded during a 480-minute, short-period thermocycling process
Fig. 4: t/CHF diagrams of seven corrosion protection primers, recorded during a 480-minute, short-period thermocycling process
First of all, it is noticeable that theCHF values obtained at 0 h are arranged in such a way that the primers with a high zinc content have high initial capacities and the zinc-free systems have low initial capacities. This is in line with the expectation that high capacitances correlate with lower impedances and therefore high transmission conductivities. For organic coatings, the initial capacitances of the high-zinc ZS systems are therefore very high; due to their high conductivity, they can no longer be regarded as a classic capacitor.
As a result of the thermocyclically induced water absorption, a clear increase in theCHF baselines is observed in the ZS systems in addition to the - initially elastic-reversible - capacitance oscillations caused by the half-hour temperature modulation. This increase indicates that a considerable amount of water penetrates the coating after a few cycles. However, water accumulation occurs in the three ZS systems at different times and to different extents. Figure 5 shows light microscope images of the three surfaces.
 Fig. 5: Light microscope images of the surfaces of the three ZS systems
Fig. 5: Light microscope images of the surfaces of the three ZS systems
It can be seen that the zinc primers, which differ only in terms of the concentration of one additive, exhibit significantly different surface porosities. The loss of water absorption reversibility (WAR) begins earlier, the more penetration possibilities the surface offers for the aqueous solution. Here, ZS2 appears to offer the least resistance to the penetrating water at first; however, its content at 40 °C apparently reaches a maximum after 300 min, which is no longer exceeded in the subsequent cycles. In contrast, although the water content in ZS0 begins to increase irreversibly at about the same time as ZS2, this takes place at a slower rate and without reaching a maximum (within the cycling time of 480 min). In ZS1, on the other hand, there is not only a strongly delayed onset of the inelastic water content oscillations, but here both the periodic extreme values and (albeit less obviously) the amplitudes reach their maximum at around 300 min. This indicates that the thermocyclically induced water diffusion paths formed up to this point are closed at a higher rate than new ones can form. It is highly probable that the reason for the closing of the diffusion paths is the cementation process mentioned above, which causes a reduction in the volume of water in the coating due to the formation of voluminous zinc oxidation products. In contrast, the concentrations of the selected additive presented in the ZS0 and ZS2 formulations result in poorer surface homogeneity and lead to the cementation effect occurring subsequently either not contributing sufficiently or not at all to the displacement of the thermocyclically induced absorbed water from the coating.
The two Zx systems differ in that with ZxB part of the zinc pigment mixture has been passivated by an organic surface treatment. This means that less active zinc pigment is available on a short time scale in ZxB than in ZxA. Consequently, ZxA has a higher initial capacitance (higher through conductivity) and greater activity (although very low compared to the ZS systems) than ZxB in the thermocycling process.
However, the smallest amplitudes - and the greatest reversibility with regard to water absorption - are consistently found in the Zad primers, which do not contain elemental zinc and whose effectiveness is essentially dependent on a high barrier. These two systems, whose matrix continuity is not disturbed by zinc pigments, consequently exhibit very high water absorption reversibility within the exposure period.
Summary:
The motivation for producing zinc primers with a reduced zinc content is to reduce the environmental impact, improve adhesion and reduce the sensitivity of adhesion under unfavorable application conditions. In principle, promising zinc primers with reduced zinc content could be obtained by partially:
- Modifying the size, shape and composition of the zinc pigment
- Providing the zinc pigment with an (organic) OF treatment
- Substitution of the zinc pigment with particulate inert conductivity additives.
These modifications influence the corrosion protection properties of the resulting (test) primers and offer the prospect of adjusting the electrochemical activity and extending the phase of cathodic corrosion protection (pcp).
EOC monitoring during thermocyclic-electrolytic exposure allows the determination of the parameter tbp, which can be regarded as a meaningful short-term test indicator of the corrosion protection properties of zinc primers.
By means of the detection of water absorption reversibility (WAR), comprehensible, additional information can be obtained about the protective effect mechanism of (zinc) primers during thermocyclic water exposure.
Funding reference:
The CORNET projects IPOC (IGF 155 EN) and ZINC-POWER (IGF 192 EN) were funded via the AiF within the framework of the program for the promotion of joint industrial research (IGF) by the Federal Ministry for Economic Affairs and Energy on the basis of a resolution of the German Bundestag.
We would like to thank the companies CONMET GmbH, MIPA AG, ECKART GmbH, The Sixth Element (Changzhou) materials Technology Co., Ltd. and Chemische Industrie Erlangen GmbH for providing pigments and binders.
Literature
[1 ]S. Feliu; R. Barajas; J.M. Bastidas; M. Morcillo: Mechanism of Cathodic Protection of Zinc-Rich Paints by
Electrochemical Impedance Spectroscopy, I. Galvanic Stage, J. Coat. Techn., 61, 775 (1989), 63-69
[2 ]S. Feliu; R. Barajas; J.M. Bastidas; M. Morcillo: Mechanism of Cathodic Protection of Zinc-Rich Paints by
Electrochemical Impedance Spectroscopy, II Barrier Stage, J. Coat. Techn., 61, 775 (1989) 71-76
[3] U. Christ; R. Nothhelfer-Richter; M. Wanner; T. Schauer: In-depth analyses, Eur. Coat. J., 5 (2010), 17-21
[4] M. Wanner; T. Schauer: New testing techniques for characterizing the protective effect and resistance
of polymeric coatings, Rubber, Fibers, Plastics, 4, (2012), 225-230


