In industrial production, chips of different shapes and sizes are distributed on the component surface during machining by means of cooling lubricants and adhere there due to electrostatic charges as well as oil and grease components. Foreign particles can be safely removed from the machined material using electrogalvanic dry steam cleaning (HD steam) without chemical cleaning additives and with lower energy consumption compared to aqueous-alkaline cleaning. The method is suitable for base materials made of ferrous metals, ceramics, plastics and non-ferrous metals everywhere in industry. The first part of a new electroplating series starts with the dirt layer structures, the material-specific properties and the electrochemical surface cleaning process.
Basically, all surfaces in mechanical and plant engineering are contaminated. All industrial objects exist together with at least one other object in the room. In addition to the gravitational force, there are attractive forces between all objects in space, caused by electrostatic charges of the individual object. Every movement of objects changes the electrical charges of the surfaces.
The technical surfaces are usually covered with particles of different origins and different physical/chemical properties. Until now, the separation of foreign particles from a surface has been carried out using alcohol-based solutions for oils and greases. Due to their electron charge, mineral foreign particles are separated galvanically or quasi-galvanically by discharging and increasing the distance to the base material surface. Alcohols and salts or electrolytes are dissolved in aqueous solutions. Soft water, water hardness less than 5°d(H), or DI water are often used as solvents because of the effect. The result is more or less well removed foreign particles in the solution and a cleaned surface of the base material with a film or crust as passivation protection.
The contaminated cleaning solution must be treated. It can often have hazardous waste properties. This is associated with costs and time as well as environmental contamination at application sites. Reuse of the contaminated solvent can only be achieved through energy and physical/chemical filtration efforts. The aim of this series of articles is to demonstrate a cleaning technology for metallic surfaces that does not have the disadvantages mentioned above. The energy expenditure is significantly reduced compared to previous applications and the valuable water as a solvent is moved in a closed cycle.
Structures of dirt layers and their properties
The component surface consists of a material, in exceptional cases of mechanically joined different materials. Each material has its own physical and chemical properties. These properties are unique and therefore significant for detection in the analysis. The behavior of the materials in relation to each other results from the properties. Foreign particles adhere to the surface of the component with varying degrees of adhesion; as the distance to the base material decreases, the binding force between the foreign particles and the base material surface increases. The contamination on the base material has a layered structure. Chemical and physical properties are assigned to each layer (Table 1).
|
Type of layer |
Layer thickness in mm |
|
Base material, not deformed, not machined |
Component |
|
Deformed boundary layer, deformed material structure |
< 0,001 |
|
Reaction layer Can be removed by HP steam |
0,000 001-0,000 01 |
|
Adsorption layer Can be removed by high pressure steam |
0,000 000 1-0,000 001 |
|
Contamination layer This layer should be removed by cleaning |
> 0,001 |
The deformed boundary layer consists of base material that has changed its microscopic structure of the crystal lattice as a result of mechanical forming and/or machining processes. Dislocations and grain size changes can occur. This layer remains unchanged in the cleaning process.
The reaction layer consists of chemical reaction products of the base material with its environment during the manufacturing process. These are usually oxides that can be removed by reduction processes. Pickling or mechanical removal by blasting processes are also conceivable. On non-machined surfaces, reaction layers are desirable as a protective coating for the base material. This layer should remain unchanged during the cleaning process. Reduction is possible when cleaning with superheated steam.
The adsorption layer in the nanometer range consists of foreign substances that do not originate from the base material. The binding energy is many times higher than in the impurity layer. If this layer is broken up and removed, e.g. by high mechanical energy, a new adsorption layer is formed immediately after the cleaning process from foreign particles in the environment. The composition of this layer can be influenced to a limited extent during the cleaning process.
The outer layers visible to the human eye are bound to the component surface by adhesion forces. Adhesion through mechanical and purely chemical bonds is not considered here.
Van der Waals forces, electrostatic forces, magnetic forces, capillary forces, other surface forces and chemical bonds are used as models to quantitatively determine the forces. If the impurities are to be removed, the binding energy must be canceled or overcome. Removing the binding energy, e.g. by discharging, generally requires considerably less energy than mechanically overcoming the binding energy by means of very high jet forces and very high kinetic energy.
In physisorption, the adsorbent is only bound to the surface by van der Waals interactions, i.e. only by physical forces. Van der Waals interactions have a large range, but are very weak. The adsorption enthalpy of a particle is typically in the order of 20-40 kJ/mol. The secondary valences are characterized by dipole-dipole bonding, hydrogen bonding, London- or dispersion bonding.
In contrast, chemisorption occurs when the bond between the particles and the surface has more covalent bond components. However, chemisorption differs from a chemical bond in that the chemisorbed particles can be mobile on the surface of the adsorbent. The adsorbed particles can be randomly distributed on the surface or in a strictly geometric order corresponding to the structure of the adsorbent surface. The adsorption enthalpy of chemisorption is much greater than that of physisorption. In chemisorption, adsorption enthalpies occur in the order of magnitude of the chemical bond enthalpies. Typical values are around 200 to 400kJ/mol. Apart from special cases, chemisorption is always exothermic [1]. The main valences are characterized by ionic bonding, atomic bonding and metal bonding.
The article presents investigations and solutions for removing the above-mentioned impurity layers. The goal is achieved by removing the reaction layer and the adsorption layer using superheated steam and galvanic discharge. The contamination layer visible to the human eye and disruptive to the production process, the outer dirt layer, is removed with this cleaning technology without chemical additives in a closed media circuit using superheated steam. The foreign particles are collected in the filter bag and in the settling basin of the collection tank as well as in the sludge of the steam generator.
The HP steam cleaning process investigated differs significantly from the carcher system. The carcass system generates hot steam of up to 200 °C in the steam generator. This steam is released onto the surface via the normal outlet nozzle; at a distance of more than 15 mm between the nozzle outlet and the surface, the steam temperature is below 70 °C; it is wet steam interspersed with water droplets. The electrical charge and the temperature are strongly dependent on the distance. A large volume flows through the outlet nozzle. The component surface is not dry after cleaning, the detached dirt film is distributed over the component surface and its surroundings. The result of cleaning by hand by repeatedly cleaning the same regions is only sufficient to a limited extent; the surrounding area is irregularly contaminated by the dissolved foreign particles.
The high-pressure steam process investigated generates steam at up to 210 °C in a special steam generator. The steam is conveyed to the HP steam nozzle, the tool, via a non-electrically conductive HP steam line. Due to its special design, the HP steam nozzle has a small outlet cross-section and therefore a low volume of used steam. The steam flows out of the nozzle at the speed of sound. The nozzle is connected as a + - pole. Adjustable hot air generated by hot air heaters up to 400 °C is drawn in by injection. The HP steam is thus superheated. As dry, electrically charged steam, the HP steam generates temperatures of up to 180 °C on the component surface. The water film on the component surface splits into H+ and OH- ions and hydrogen peroxide. By applying suction pressure, an injector nozzle driven by steam, the steam vapors with the dissolved dirt particles are completely extracted and condensed to 80 °C in the collection tank. The electrical charge of the condensate is ideal for filling the steam generator. A closed water circuit minimizes water consumption and energy consumption.
Objects have material-specific physical properties
Metallic materials have a lower ionization energy than non-metals. When energy is supplied, at least 1 electron leaves the shell. This process is the beginning of the ionization of a metal atom. These free electrons do not belong to an electron shell of neighboring atoms, they are freely movable. The metal bond is based on the electrostatic interaction between the metal cations, often referred to in this context as positive atomic trunks, and electrons (conduction electrons) moving in the solid state. With a binding energy of approx. 200 kJ/mol, the metal bond is one of the weaker main valences. In analogy to gas theory, the so-called "freely" moving electrons are also called electron gas.
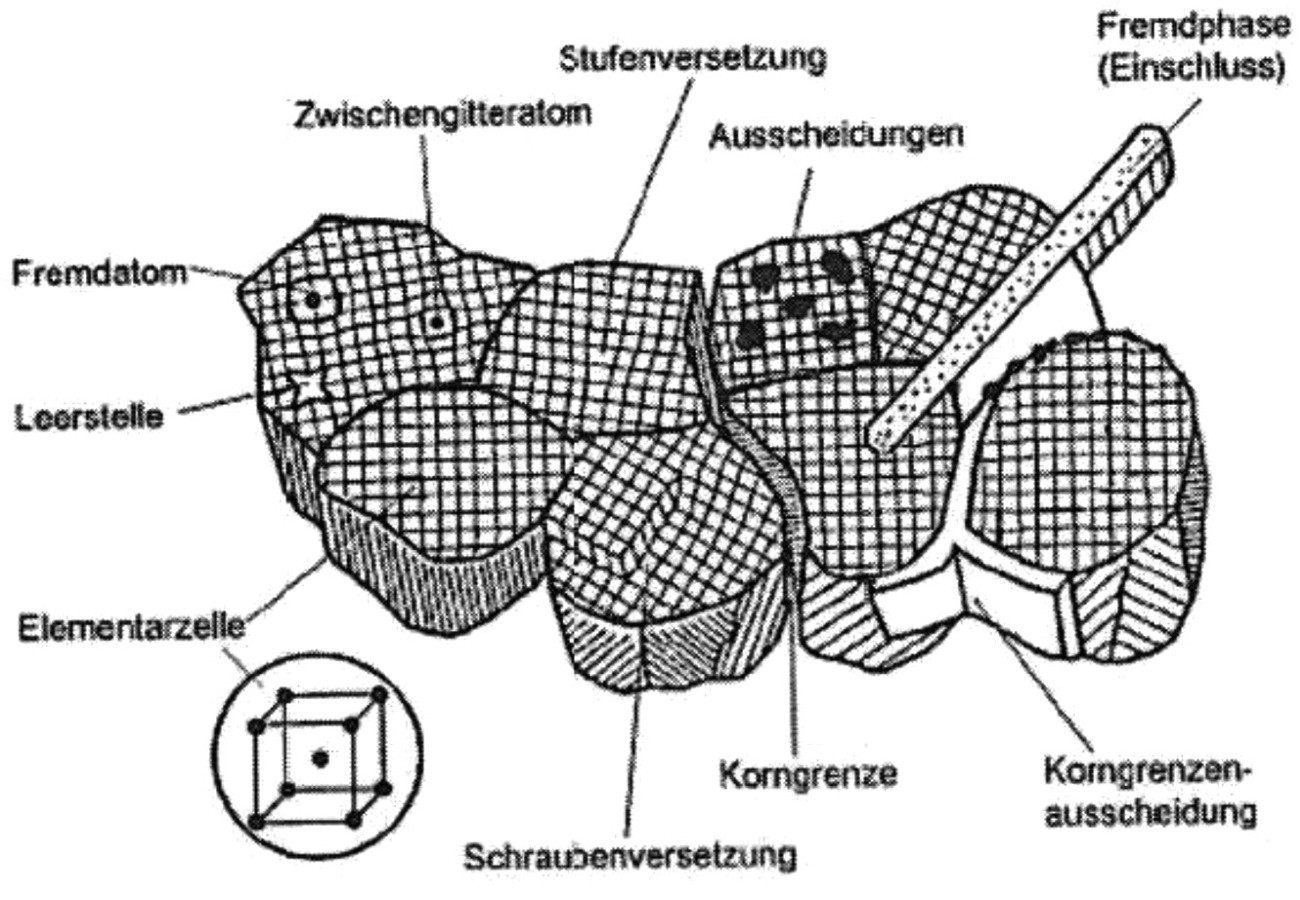 Fig. 1: Lattice defects in metals
Fig. 1: Lattice defects in metals
The metal bond is a non-directional bond. A maximum packing density can therefore be achieved in a volume element of the metallic solid. The familiar lattice structures are formed, such as face-centered cubic, body-centered cubic and hexagonal. In terms of structure, metals are crystalline materials whose building blocks are three-dimensional, periodically repeating, in the form of a long-range order. This corresponds to the idea of an ideal lattice. The real lattice deviates from this model and has lattice faults (defects) such as
- Point defects (vacancies, interstitial atom, foreign atom)
- Line defects (dislocations)
- Surface defects (grain boundaries, phase boundary, etc.)
- Volume defects (inclusions, precipitates, etc.), which are illustrated in Figure 1.
Any deviation from the ideal metal lattice causes a deviation from the equilibrium distance and thus leads to the formation of a stress state in the atomic range. This has a particular effect on the mechanical behavior of the metals and to a significant extent on the electrical conductivity. Lattice defects also influence the corrosion behavior [2].
In most cases, the metals used in practice are polycrystalline. The grain boundary, an area with disturbed interatomic order, represents an area with increased energy content. While the bonds between the atomic bodies inside the metal lattice are saturated, this does not apply to the surface or the area close to the surface. They still have bonding electron states on the outside (surface free energy) and cause the formation of bonds with available atoms, ions or molecules at the interface between the metal surface and the environment (see Fig. 2).
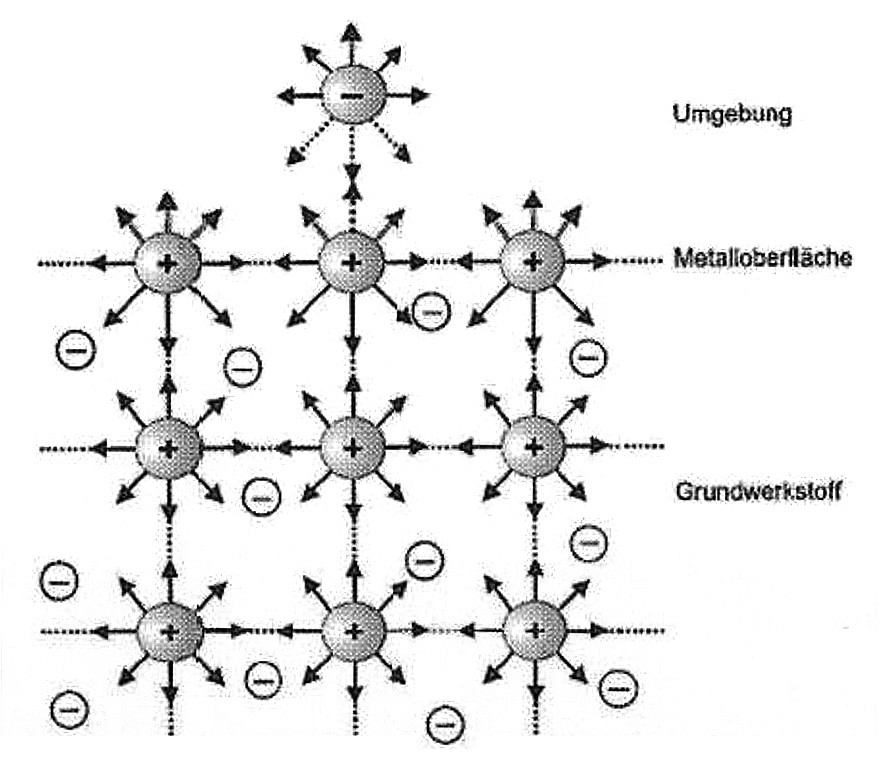 Fig. 2: Interaction of the free metal surface with other media
Fig. 2: Interaction of the free metal surface with other media
For example, water is electrostatically bound to the metal surface as a dipole molecule. As the water molecule is not an ion, i.e. it does not carry a full elementary charge, it does not form a main valence in the sense of an ionic bond, but a secondary valence bond. The binding energy is approx. 30 kJ/mol.
Any processing of the metal leads to lasting changes in structure and microstructure. Figure 3 shows a pictorial representation of the real workpiece surface after machining.
In addition to shaping, the consequences of machining must also be taken into account:
- Change in the electrochemical potential
- Hardening
- Anisotropic behavior with texture
- Change in the surface profile.
The electrochemical potential changes to negative values, making the surface more susceptible to corrosion. For example, the deformation edge zone must be removed from a high-alloy chromium-nickel steel with high corrosion resistance in order to restore the original corrosion resistance, e.g. by electrochemical polishing.
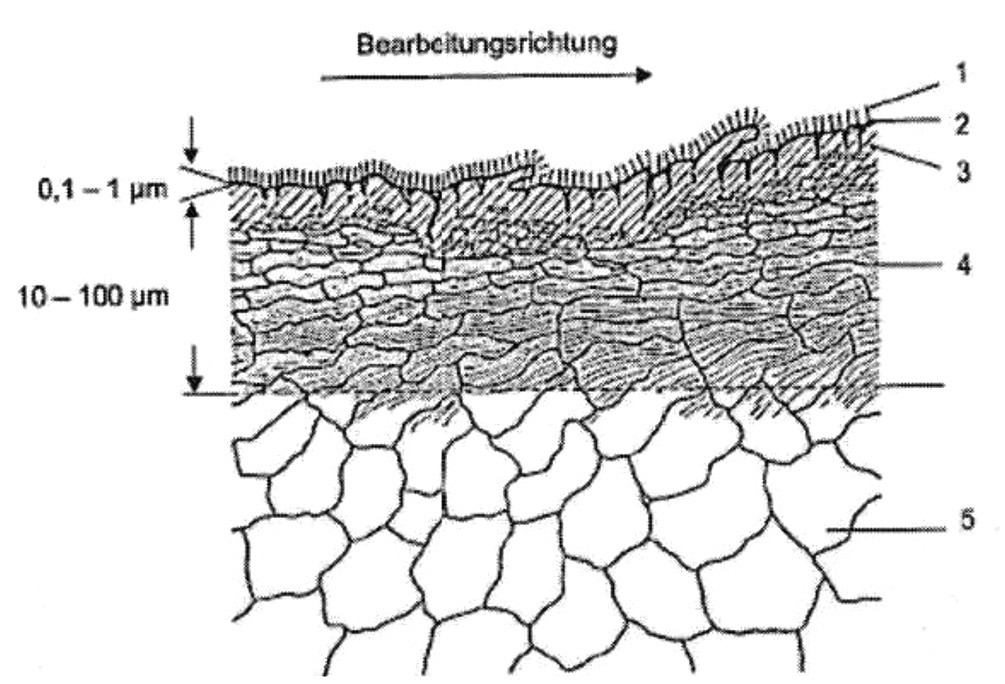 Fig. 3: Example of the machined workpiece surface of a metal. Legend: 1) Grease, cooling lubricant or oil film with solvent, 2) adsorption and reaction layer, 1+2 form the outer boundary layer, 3) transition zone, 4) inner boundary layer, 5) undisturbed metal structure
Fig. 3: Example of the machined workpiece surface of a metal. Legend: 1) Grease, cooling lubricant or oil film with solvent, 2) adsorption and reaction layer, 1+2 form the outer boundary layer, 3) transition zone, 4) inner boundary layer, 5) undisturbed metal structure
During machining, chips are produced that have a different charge from the base material. As a rule, the chips are attracted to the base material. Together with the cooling lubricants, the loose chips then "stick" more or less securely to the component surface, to the base material. This "adhesive bond" must be broken and the loose, free chips must be disposed of and safely separated from the component. The aim must be to hand over the component surface free of chips and free of foreign films for further production. Applying Archimedes' principle of buoyancy significantly reduces the kinetic energy required for displacement. A body loses as much weight in a liquid as the liquid it displaces weighs. For example, water as a solvent is energetically more favorable than oil.
Processes during the electrochemical cleaning of surfaces
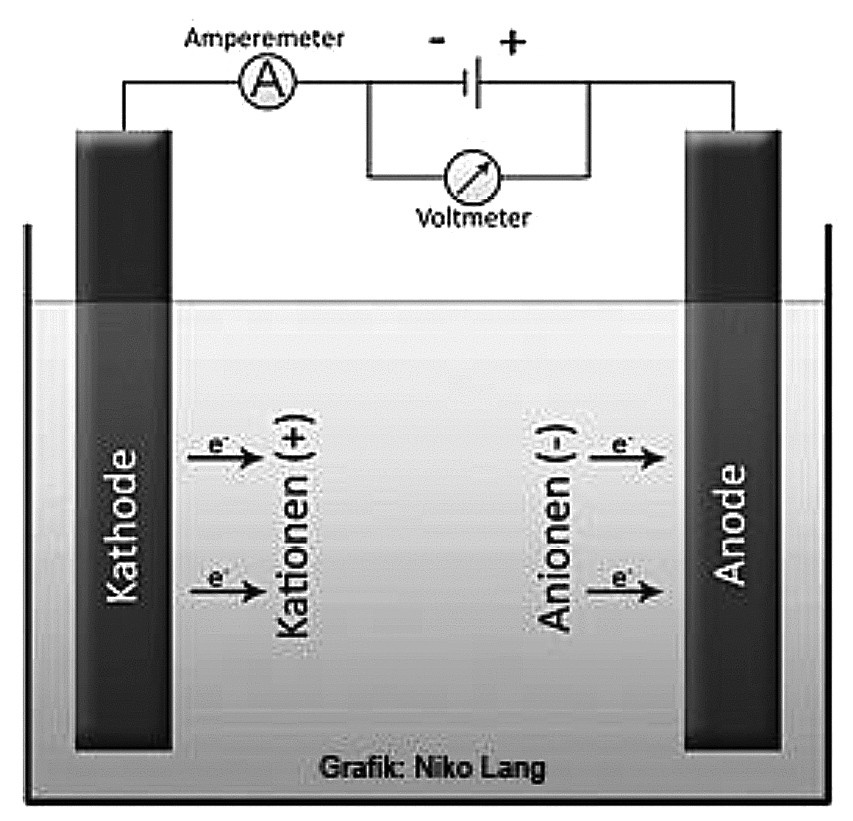
In electrolysis, which is shown in Figure 4, electrical energy is converted into chemical energy.
A direct electric current flows between an anode (+) and a cathode (-) in a conductive electrolyte liquid. Reaction products are formed on the surfaces of the electrodes from the substances contained in the electrolyte and the materials of the electrode. The voltage source (usually direct current between 2 and 3.5 volts) causes a lack of electrons at the positive pole (anode) and an excess of electrons at the negative pole (cathode). The positively charged cations migrate to the negatively charged cathode. At the cathode, they take up electrons and are thus reduced. The opposite process takes place at the anode.
If the component to be processed is switched to anode (+) and the cleaning electrode to minus (-), the selected electrolyte is used to remove material and contamination from the component. The component surface is cleaned of foreign particles and polished, as component particles are removed (fine tips, sharp edges). Roughness peaks of the component are removed more than the roughness valleys, the surface is polished in the nano range and the oxides are removed (rust removal). Pulsating alternating circuits dose the possible removal. At the end of processing, the surface must be passivated. In the case of metals, chromium should be deposited due to oxide formation.
If the component is connected as a cathode, dissolved material from the electrolyte solution is applied to the component surface. During electrochemical cleaning, the protective oxide layer builds up due to the oxygen released from the water. Dissolved ions are deposited. Special carbon is used as the cathode [3]. Alternatively, the electrolysis bath can be electrically charged. With a DC voltage of between 2 and 3.5 volts, the electrodes are the insulated metal chamber/tub and the electrode freely suspended in the solution bath. The free electrode is connected as the anode. The material of the free electrode is silver plate. Dissolved ions from the bath solution are deposited on the surface of the free electrode. The galvanic solution is electrostatically charged, usually more positively. This accelerates or delays the reactions on the component surfaces. The current flow approaches zero. The voltage is decisive here. The galvanic solution can therefore contain fewer charged minerals and the electrical voltage remains constant.
Based on the above-mentioned physical and chemical reactions resulting from electroplating, the findings are transferred to wet and dry vapor. In this series of articles, the effects of steam combined with an external electrical charge between 2 and 3.5 volts DC are examined.
Part 2 will deal with the object and its dirt film as well as the task of surface cleaning
Literature
[1] http://www.chemgapedia.de/
[2] H. Hofmann; J. Spindler: Aufbau und Eigenschaften oberflächennaher Werkstoffbereiche, Verfahren der Oberflächentechnik, 15-27
[3] Documentation on the Internet, Reuter GmbH & Co. KG, Düsseldorf