Silicon (Si) is an important raw material for many industrial applications, including microelectronics, the photovoltaic industry and, more recently, electrochemical energy storage. However, adaptable deposition technologies are required to expand the technical application spectrum of Si.
![Abb. 1: (a) Foto der im Labor hergestellten Referenzelektrode. Ein Silberchloriddraht wird in das entsprechende Lösungsmittel mit 0,1 M TBACl getaucht. Die Verbindungsstelle wird durch eine Vycor- Glasfritte mit einem Porendurchmesser von 10 nm gebildet. Zyklovoltammogramme in 2,5 mM Ferrocen in [BMP][TFSI] (b) und PC + 0,1 M TBACl (c); Arbeits-/Gegenelektrode: Pt-Drähte, Scan-Rate: 10 mV s-1](/images/stories/Abo-2022-07/thumbnails/thumb_gt-2022-07-0032.jpg) Fig. 1: (a) Photo of the reference electrode produced in the laboratory. A silver chloride wire is immersed in the corresponding solvent with 0.1 M TBACl. The junction is formed by a Vycor glass frit with a pore diameter of 10 nm. Cyclic voltammograms in 2.5 mM ferrocene in [BMP][TFSI] (b) and PC + 0.1 M TBACl (c); working/counter electrode: Pt wires, scan rate: 10 mV s-1 To date, most of the available methods require high vacuum or very high temperatures [1, 2]. A less complex and cost-effective alternative for the production of Si is electrochemical deposition [3]. The great advantage of electrochemistry is the possibility of adjusting the properties of the layers accordingly by varying the deposition parameters, e.g. the electrolyte composition and the electrochemical potential. However, due to the high reactivity of the silicon precursors with water and the very low reduction potential, deposition must take place in non-aqueous media [4]. Initial attempts at the electrodeposition of silicon were made in electrolytes based on propylene carbonate (PC) [5] and tetrahydrofuran [6]. In addition, other researchers have reported the successful reduction of silicon from acetonitrile [7-9], tetrahydrofuran [8, 10], propylene carbonate [11-18], high-temperature molten salts (HTMS) [19, 20] and ionic liquids (IL) [1-3, 21-26]. However, the disadvantages of conventional organic media are their high vapor pressure and high flammability, so that appropriate safety precautions must be taken when using them as electrolytes. HTMS are also challenging as electrolytes because they are extremely reactive and require a lot of energy. In addition, deposition from propylene carbonate or acetonitrile leads to silicon layers with a considerable amount of carbon and oxygen impurities due to the decomposition of the solvent during the reduction process [11]. In addition to conventional organic solvents, ILs have attracted great interest due to their high stability and wide potential window [1, 24]. These salts, which are liquid at room temperature, have low toxicity, low vapor pressure and are non-flammable. Nevertheless, it was found that deposition from ILs is accompanied by the incorporation of solvent molecules and decomposition products into the silicon layers [24, 27, 28]. El Abedin and coworkers demonstrated the possibility of depositing silicon thin films from these solvents as early as 2004 [1]. In 2013, the same group analyzed the influence of different anions of ILs on the film properties at different temperatures [29]. Vlaic et al [24] and Komadina and coworkers [27] used electrochemical quartz crystal microbalance (EQCM) and X-ray photoelectron spectroscopy (XPS) to prove that chemical side reactions also take place during the deposition process. This could be an explanation for the detection of decomposition products of the IL in the layers. A more detailed insight into the processes at the electrode-electrolyte interface at the molecular level was recently provided by Tsuyuki et al [30]. Using X-ray reflectance measurements and density functional theory (DFT) calculations, they found that a polymer-like structure of SixCly components forms at the electrode surface during reduction. A large number of different deposition parameters were investigated by Thomas and coworkers [31]. It was found that overvoltage and temperature in particular have a decisive influence on silicon deposition.
Fig. 1: (a) Photo of the reference electrode produced in the laboratory. A silver chloride wire is immersed in the corresponding solvent with 0.1 M TBACl. The junction is formed by a Vycor glass frit with a pore diameter of 10 nm. Cyclic voltammograms in 2.5 mM ferrocene in [BMP][TFSI] (b) and PC + 0.1 M TBACl (c); working/counter electrode: Pt wires, scan rate: 10 mV s-1 To date, most of the available methods require high vacuum or very high temperatures [1, 2]. A less complex and cost-effective alternative for the production of Si is electrochemical deposition [3]. The great advantage of electrochemistry is the possibility of adjusting the properties of the layers accordingly by varying the deposition parameters, e.g. the electrolyte composition and the electrochemical potential. However, due to the high reactivity of the silicon precursors with water and the very low reduction potential, deposition must take place in non-aqueous media [4]. Initial attempts at the electrodeposition of silicon were made in electrolytes based on propylene carbonate (PC) [5] and tetrahydrofuran [6]. In addition, other researchers have reported the successful reduction of silicon from acetonitrile [7-9], tetrahydrofuran [8, 10], propylene carbonate [11-18], high-temperature molten salts (HTMS) [19, 20] and ionic liquids (IL) [1-3, 21-26]. However, the disadvantages of conventional organic media are their high vapor pressure and high flammability, so that appropriate safety precautions must be taken when using them as electrolytes. HTMS are also challenging as electrolytes because they are extremely reactive and require a lot of energy. In addition, deposition from propylene carbonate or acetonitrile leads to silicon layers with a considerable amount of carbon and oxygen impurities due to the decomposition of the solvent during the reduction process [11]. In addition to conventional organic solvents, ILs have attracted great interest due to their high stability and wide potential window [1, 24]. These salts, which are liquid at room temperature, have low toxicity, low vapor pressure and are non-flammable. Nevertheless, it was found that deposition from ILs is accompanied by the incorporation of solvent molecules and decomposition products into the silicon layers [24, 27, 28]. El Abedin and coworkers demonstrated the possibility of depositing silicon thin films from these solvents as early as 2004 [1]. In 2013, the same group analyzed the influence of different anions of ILs on the film properties at different temperatures [29]. Vlaic et al [24] and Komadina and coworkers [27] used electrochemical quartz crystal microbalance (EQCM) and X-ray photoelectron spectroscopy (XPS) to prove that chemical side reactions also take place during the deposition process. This could be an explanation for the detection of decomposition products of the IL in the layers. A more detailed insight into the processes at the electrode-electrolyte interface at the molecular level was recently provided by Tsuyuki et al [30]. Using X-ray reflectance measurements and density functional theory (DFT) calculations, they found that a polymer-like structure of SixCly components forms at the electrode surface during reduction. A large number of different deposition parameters were investigated by Thomas and coworkers [31]. It was found that overvoltage and temperature in particular have a decisive influence on silicon deposition.
In the last two decades, great interest has been shown in silicon as an anode material for lithium-ion batteries (LIB) [32, 33]. The capacity of the commonly used anode - graphite - is limited to 372 mAh/g and therefore needs to be replaced by another material to increase the energy density of LIBs [34]. Besides tin [32, 33, 35] and lithium [36-73], silicon is a promising candidate as it has a high theoretical capacity of 4.2 Ah g-1 for Li22Si5 [13] and a low charging voltage, similar to graphite anode [12]. However, lithiation of this material is associated with high volume expansions (more than 300 %), which leads to a rapid capacity drop [14]. Approaches to solve these challenges are Si nanomaterials, nanocomposites and nanostructuring of the electrodes [38, 39]. Titanium dioxide nanotubes offer both space for the volume increase of silicon during lithiation and a large surface area, which leads to better lithium ion diffusion [7, 40]. Coated with silicon, they are therefore a promising candidate for anodes in LIBs, especially for microbattery applications. Various electrolyte systems, both water-based and with organic solvents, have already been investigated for the production of TiO2 nanotubes [41-43]. The most promising results have so far been obtained using glycerol or ethylene glycol as solvent and HF or NH4F as fluoride source [39, 41, 44]. Ivanov et al [45] and Brumbarov and coworkers [44], who sputtered silicon onto the nanotubes to improve the cycling stability, have reported initial success in the application in LIBs. Nanostructured copper electrodes are an alternative to TiO2, as copper has a higher electrical conductivity than TiO2. In addition, it is already used as a stable current collector in lithium-ion batteries and the production of copper foam is simple and inexpensive.
Another problem is the high irreversible capacity loss in the first cycle when silicon is used in LIBs [37, 46-51]. Rehnlund et al. recently reported a lithium ion trapping mechanism in silicon materials that is responsible for this phenomenon [35]. One way to circumvent this problem could be the use of pre-lithiated anode materials [50].
Recycling of used electrolytes, especially ILs, is an important issue due to their high cost. It is generally known that ILs partially decompose when heated, which leads to a color change [52]. Since even the smallest amounts of impurities affect the physical and chemical properties, efficient purification techniques must be developed [53]. Various methods are used to improve the purity of these solvents, such as distillation [52, 54], extraction [54], zone melting [52] or membrane-based processes [54]. All these methods have advantages and disadvantages. In addition, it depends strongly on the properties of the IL, e.g. whether it is miscible with water or not, and on the expected impurities, which purification process is best suited for electrolyte recycling [54].
In addition to the results on the electrochemical deposition of silicon and the influence of different substrates and electrolytes on the thin films, this article presents a simple way of using a reference electrode for different organic solvents. In addition, the results of the investigation of nanostructured substrates and finally an experiment on the recycling of the ionic liquid used are shown.
Experiments Chemicals and materials
High purity propylene carbonate and silicon tetrachloride were purchased from Alfa Aesar. 0.1 M tetrabutylammonium chloride (Sigma Aldrich, purity ≥ 97 %) served as the conducting salt. The mixture of PC and tetrabutylammonium chloride (TBACl) was dried with a molecular sieve (pore diameter 0.3 nm, CarlRoth, Germany) for two days before the addition of the silicon precursor. The ionic liquid 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide [BMP][TFSI] (99 %) was selected due to its wide potential window and purchased from IoLiTec (Heilbronn, Germany) [1, 24]. It was dried under vacuum at 120 °C for 48 h until a water content of less than 15 ppm was reached. The moisture in the solvents for the electrochemical experiments was determined by Karl Fischer titration using a Metrohm 831 KF coulometer.
The chemicals for the anodization process (glycerol (99 %), NH4F (98 %)) of titanium foils (96 %, 0.25 mm (GoodFellow)) were also purchased from Alfa Aesar.
Reference electrode
A laboratory-prepared Ag/AgCl electrode with organic electrolyte, based on an idea by Saheb et al [55], was used as the reference electrode. A silver wire was oxidized with a saturated FeCl3 solution for 3 minutes, rinsed with water and dried under vacuum. It was then inserted into a 6.4 cm long glass capillary containing a solution of 0.1 M TBACl in [BMP][TFSI] or PC and sealed with a glass frit (Vycor glass, pore diameter 10 nm) on the opposite side. The electrodes were calibrated against the redox couple ferrocene/ferrocenium (fc/fc+) in the corresponding solvent (2.5 mM fc in [BMP][TFSI] or PC + 0.1 M TBACl).
Electrochemical deposition of silicon
All experiments were carried out in a glovebox filled with argon (MBraun UNIlab LMF auto), with the water and oxygen content each kept below 0.1 ppm. A self-made cell made of PTFE was used for the electrochemical measurements. The working electrode (Cu or Ni) is clamped to the cell via a Viton O-ring, resulting in a geometric area of 0.33 cm2. A platinum plate (A = 2 cm2) served as a counter electrode and was heated to red heat before the silicon electrolyte deposition. All potentials were measured against the reference electrodes mentioned above. Copper and nickel were polished with a silicon carbide abrasive paper (4000 grit), rinsed with ethanol and dried under argon for one hour. Electrolytes were prepared by dissolving 0.5 M SiCl4 in [BMP][TFSI] or PC and stirred for at least 12 hours. All electrochemical measurements were performed using a multichannel potentiostat/galvanostat (VMP3 Bio-Logic Scientific Instruments).
After deposition, the samples were thoroughly rinsed with PC to remove residual electrolytes.
Anodization of titanium
Before synthesizing the TiO2 nanotubes, the titanium foils were polished with SiC sandpaper (4000 grit) and cleaned for 5 minutes in ethanol or acetone in an ultrasonic bath. The anodization process was carried out in an electrochemical cell (two-electrode configuration) with a Pt grid as counter electrode (40 V for 75 min). The electrolyte consisted of 0.5 wt% NH4F and 2.0 wt% H2Oin glycerol. Further details on the anodization of Ti can be found in [41].
The electrochemical reduction of the nanotubes was carried out in H2SO4, K2SO4 and KOH (each E = -1.5 V vs. Ag/AgCl (total KCl)) at a concentration of 0.5 M for 1 minute each.
Surface analysis
The surface morphology of all samples was analyzed using an ultra-high resolution scanning electron microscope (FE-SEM, Hitachi S-4800) with EDX analysis.
Recycling of [BMP][TFSI]
The recycling of ILs was performed similarly to the purification steps after the synthesis of [BMP][TFSI] [56]. The spent electrolyte was stirred for 3 hours with twice the amount of distilled water (Millipore-Milli-Q system, resistance 18.2 MΩ) to remove residual SiCl4. After a filtration step to remove solid particles (SiO2), water and IL were separated by a separatory funnel and collected in extra bottles. The IL was washed 6 times with H2O(ratio 1:4). Subsequently, traces of the ionic liquid in the wash water were extracted with dichloromethane (H2O: CH2Cl2 = 4:1) and mixed with the remaining IL to reduce the viscosity for the next step. To remove organic impurities, the solution of [BMP][TFSI] and dichloromethane was filtered three times through a glass column with frit (G4) filled with granulated activated carbon. After microfiltration (pore diameter 0.2 µm), the CH2Cl2 was removed with the rotary evaporator. The remaining [BMP][TFSI] was dried under vacuum at 120 °C for 48 hours. The measurement of the electrochemical stability window was carried out in an intert atmosphere with a working and counter electrode made of platinum (geometric area 0.018 cm2 and 2 cm2 respectively).
Results and discussion Reference electrodes
In almost all studies on silicon deposition, Pt was used as a quasi-reference electrode. However, these electrodes are not known to provide a stable potential, as can be seen from the potential variation of the silicon reduction from -2.0 V [24] to approx. -2.4 V [29]. In addition, the potential of the Pt electrode often shifts during the experiments [4]. A reference electrode with a higher stability is required for better comparability of the results and especially for kinetic measurements. Therefore, an Ag/AgCl electrode with organic electrolyte (Fig. 1a) is used in the experiments. We have found that the potential of this electrode changes with its geometry. Therefore, calibration against an internal standard is absolutely necessary. A well-known and frequently investigated redox couple is ferrocene/ferrocenium. In most organic solvents, including ILs and PC, it shows good reversibility, although the peak-to-peak distance is greater than the ideal 57 mV for a reversible one-electron process [55, 57-61] at 298 K. It is assumed that this is due to the uncompensated resistance in the electrolyte [61]. Therefore, all voltammetric measurements were performed using the internal IR compensation of the potentiostat.
The reference potentials were calculated according to equation (1):
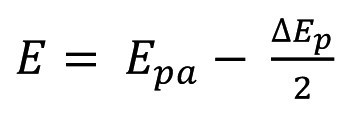
Where E is the calculated reference potential of the electrode, Epa is the peak potential of the oxidation process and ΔEp is the peak-to-peak distance [61]. The calculated values are 503 +/- 2 mV for the IL-based reference electrode and 714 +/- 5 mV for the PC-based reference electrode.
From the cyclic voltammograms in Figure 1b it can be seen that the IL-based electrode is stable for up to five months. After this time, the Vycor glass frit was clogged, but could be used again after cleaning with H2O2. The differences in the currents are due to the different immersion depths of the Pt wire used for calibration.
In contrast, the potential of the PC-based reference shifted by 25 mV to more positive values within 5 weeks (Fig. 1c), which is why it was replaced every third week.
Electrochemical deposition of silicon
It is known that silicon deposition from SiCl4 in organic media is irreversible [6, 15, 29]. To investigate the reduction process and determine the potential for silicon deposition, LSVs were measured. In [BMP][TFSI], three reduction peaks can be observed at -0.6 V, -1.15 V and -1.6 V for the copper substrate (Fig. 2, black line). The latter peak varies between -1.6 V and -1.7 V depending on the pretreatment of the substrate and corresponds to the deposition of silicon in agreement with the literature [29]. The first and second peaks are attributed to adsorption of IL, underpotential deposition/alloy formation [29] or partial reduction of Si4+ to Si2+. Density functional theory (DFT) calculations by Tsuyuki et al. indicate that a partial reduction of the precursor takes place, where first a Si2Cl6 dimer is formed and upon further reduction a polymer-like structure is formed in front of the electrode surface [30]. However, they used an IL with ammonium cation for their experiments. Nevertheless, a similar process is also conceivable for [BMP][TFSI]. This would mean that the first two peaks could be adsorption and/or partial reduction of the silicon chloride with subsequent polymer formation. At potentials more negative than -2.0 V, decomposition of the electrolyte occurs.
![Abb. 2: Linear Sweep Voltammetrie, gemessen in [BMP][[TFSI] mit 0,5 M SiCl4 bei einer Scan-Rate von 10 mV s-1. Einschub: REM-Aufnahmen der Siliciumschichten auf Kupfer (a, b) und Nickel (c, d), die durch potentiostatische Abscheidung bei -1,7 V für 2 h erhalten wurden Abb. 2: Linear Sweep Voltammetrie, gemessen in [BMP][[TFSI] mit 0,5 M SiCl4 bei einer Scan-Rate von 10 mV s-1. Einschub: REM-Aufnahmen der Siliciumschichten auf Kupfer (a, b) und Nickel (c, d), die durch potentiostatische Abscheidung bei -1,7 V für 2 h erhalten wurden](/images/stories/Abo-2022-07/gt-2022-07-0033.jpg) Fig. 2: Linear sweep voltammetry measured in [BMP][[TFSI] with 0.5 M SiCl4 at a scan rate of 10 mV s-1. Inset: SEM images of the silicon layers on copper (a, b) and nickel (c, d) obtained by potentiostatic deposition at -1.7 V for 2 h
Fig. 2: Linear sweep voltammetry measured in [BMP][[TFSI] with 0.5 M SiCl4 at a scan rate of 10 mV s-1. Inset: SEM images of the silicon layers on copper (a, b) and nickel (c, d) obtained by potentiostatic deposition at -1.7 V for 2 h
A different shape of the voltammetric curves can be observed for the nickel substrate (Fig. 2, red line). Two peaks (-0.4 V and -1.7 V) and two waves (-0.9 V and -1.35 V) can be seen in the voltammogram. The first peak (-0.4 V) could be an adsorption of the IL. There may also be a partial reduction of silicon on nickel, but at a different rate than on Cu. At 1.7 V, the deposition of silicon takes place. As far as we know, there is no literature on the deposition of silicon from ILs on nickel to compare the results.
In contrast, for PC (Fig. 3) there is only one reduction peak at -1.1 V for both substrates. Below 1.5 V, the electrolyte decomposes. Nevertheless, potentiostatic experiments at -1.1 V do not lead to visible layers, indicating that silicon reduction occurs at more negative potentials. In fact, the LSVs show a small wave at -1.75 V (black curve) for copper and a peak at 1.9 V for nickel. Due to the decomposition of the electrolyte, potentiostatic deposition was performed at -1.5 V. However, only a very thin layer was visible on nickel, so that further deposition experiments were carried out at a more cathodic potential. Similar results were also obtained by other researchers [13-15].
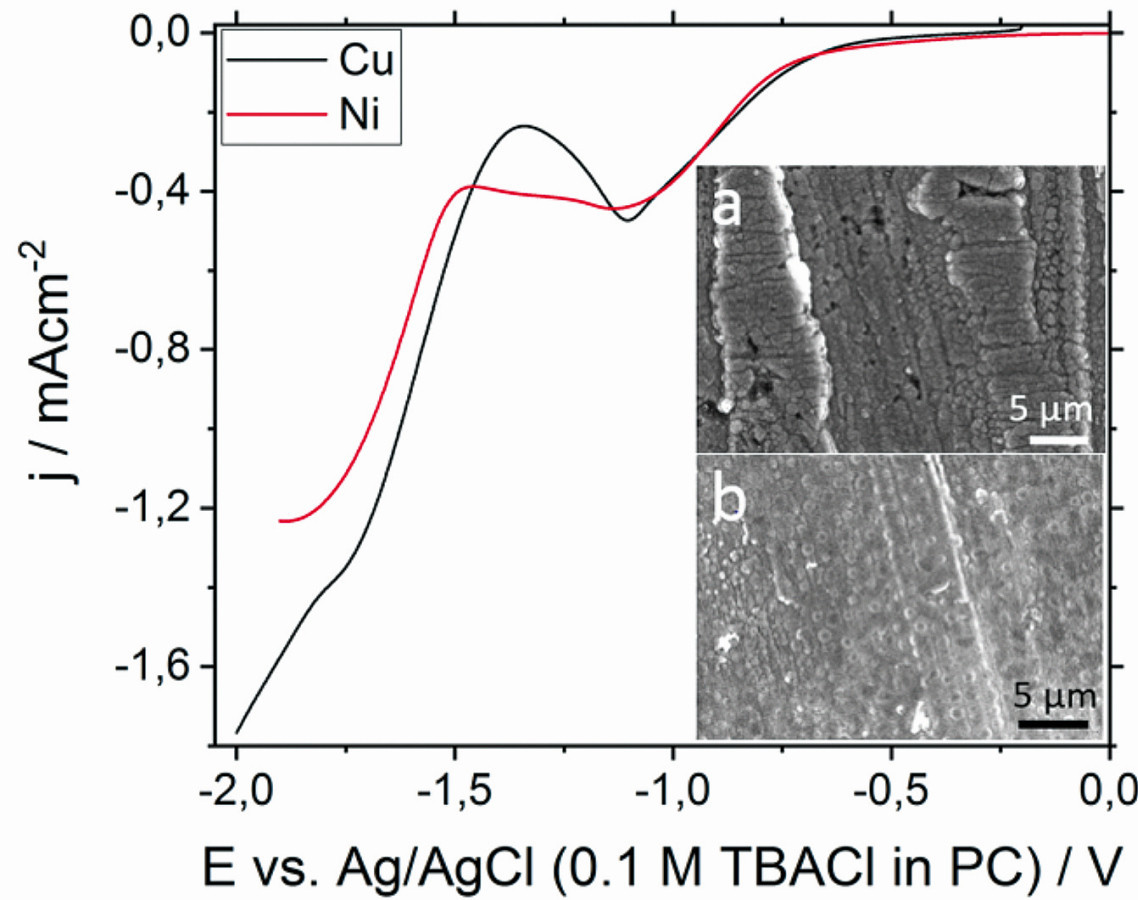 Fig. 3: Linear sweep voltammograms of 0.5 M SiCl4 in a propylene carbonate-based electrolyte (ν = 10 mV s-1). Inset: SEM images of the layers formed during deposition at -1.7 V for 2 h on copper (a) and at -1.88 V for 2 h on nickel (b)
Fig. 3: Linear sweep voltammograms of 0.5 M SiCl4 in a propylene carbonate-based electrolyte (ν = 10 mV s-1). Inset: SEM images of the layers formed during deposition at -1.7 V for 2 h on copper (a) and at -1.88 V for 2 h on nickel (b)
The influence of the different substrates and electrolytes on the silicon deposition can be analyzed by comparing the current-time transients (Fig. 4). Table 1 summarizes the parameters of the potentiostatic experiments.
|
Substrate |
Cu |
Ni |
Cu |
Ni |
TiO2 |
|
Electrolyte |
[BMP][TFSI] |
[BMP][TFSI] |
PC |
PC |
[BMP][TFSI] |
|
c (SiCl4) |
0.5 M |
0.5 M |
0.5 M |
0.5 M |
0.1 M |
|
Potential |
-1.7 V |
-1.7 V |
-1.5 V |
-1.9 V |
-1.9 V |
|
Deposition color |
Orange-brown |
Yellowish-green |
Orange-brown |
Yellowish-green |
Black |
First of all, it should be mentioned that the absolute current for the same substrate, the same electrolyte and the same applied potential is not identical due to the different active surface areas resulting from the pre-treatment. Therefore, the data presented in Figure 4 only show a trend of the current behavior. Regardless of the electrolyte type, the copper substrates (Fig. 4a, b) always show the highest currents. The higher current in PC compared to IL can be explained by the lower viscosity of this electrolyte. Nevertheless, the current of the nickel substrate in PC (Fig. 4d) is even lower than in the IL. One explanation for this could be the decomposition of the electrolyte, which hinders the diffusion of further SiCl4 molecules to the electrode surface, resulting in only a very thin layer. Similar assumptions are also made by Nishimura and coworkers [15]. In contrast, several waves are visible in the current transient for nickel in the IL-based solution (Fig. 4c). The decomposition of the electrolyte could explain this behavior.
![Abb. 4: Chronoamperogramme von Kupfer und Nickel in [BMP][TFSI] und Propylencarbonat sowie von TiO2-Nanoröhrchen in [BMP][TFSI]. Die Konzentration des Präkursors beträgt jeweils 0,5 M, außer bei den TiO2-Nanoröhren (0,1 M). Die angelegten Potentiale sind -1,7 V (a), -1,5V (b), -1,7V (c), -1,9 V (d) und -1,9 V (e) Abb. 4: Chronoamperogramme von Kupfer und Nickel in [BMP][TFSI] und Propylencarbonat sowie von TiO2-Nanoröhrchen in [BMP][TFSI]. Die Konzentration des Präkursors beträgt jeweils 0,5 M, außer bei den TiO2-Nanoröhren (0,1 M). Die angelegten Potentiale sind -1,7 V (a), -1,5V (b), -1,7V (c), -1,9 V (d) und -1,9 V (e)](/images/stories/Abo-2022-07/gt-2022-07-0035.jpg) Fig. 4: Chronoamperograms of copper and nickel in [BMP][TFSI] and propylene carbonate as well as of TiO2 nanotubes in [BMP][TFSI]. The concentration of the precursor is 0.5 M in each case, except for the TiO2 nanotubes (0.1 M). The applied potentials are -1.7 V (a), -1.5 V (b), -1.7 V (c), -1.9 V (d) and -1.9 V (e)
Fig. 4: Chronoamperograms of copper and nickel in [BMP][TFSI] and propylene carbonate as well as of TiO2 nanotubes in [BMP][TFSI]. The concentration of the precursor is 0.5 M in each case, except for the TiO2 nanotubes (0.1 M). The applied potentials are -1.7 V (a), -1.5 V (b), -1.7 V (c), -1.9 V (d) and -1.9 V (e)
As the active surface of the nanostructured substrate is much larger than the geometric surface, the current densities are difficult to compare with those of copper or nickel. The TiO2 nanotubes have a very low current, which is not only due to the lower concentration of the precursor, but also to the lower conductivity of this material. The current should be the lowest of all substrates if the exact surface area is known and used to calculate the current density.
Surface analysis of silicon thin films
The morphological characterization of the silicon deposits was carried out using SEM. The layers obtained from the IL are dense and show only a few cracks (Fig. 2 a, c). At higher magnifications (Fig. 2 b, d) a spherical surface morphology can be recognized. Spherical objects with diameters between 50 nm and 400 nm can be seen on copper. Similar observations have been reported by other researchers [24, 29]. In contrast, a superimposed structure with spherical particles is recognizable on Ni. These structures enable the inclusion of electrolyte molecules in the layer. EDX analysis of the layers showed the presence of C, O, N, F, S and Cl, which originate from trapped electrolyte or decomposition products. The stability of the [TFSI] ion during deposition was investigated in detail by Vlaic et al [24]. In addition to the electrolyte, oxygen originates from contact with air during sample transport to the SEM.
In contrast, the silicon layer obtained from PC on copper shows a superimposed and very rough structure with cavities (Fig. 3a). This agrees with the work of Momma and coworkers [11] and Epur et al [13], who observed a rough structure with cracks and uneven thickness. The thin film on nickel is also rough but has no cracks. The size of the particles varies between 100 nm and 700 nm. Similar results were obtained by Nishimura and Fukunaka [15]. However, they used galvanostatic methods or much higher potentials (-3.6 V vs. Pt-QRE). In addition to silicon, carbon, oxygen and a small amount of chlorine are also found in these layers.
The exact composition of the silicon layers is currently being investigated using X-ray photoelectron spectroscopy (XPS).
Nanostructured substrates Titanium dioxide nanotubes
The use of nanostructured electrodes, in particular TiO2 nanotubes, could buffer the strong volume expansion of silicon during lithiation. However, titanium dioxide has a very low electrical conductivity, which could pose a problem for silicon deposition. Therefore, we tried to increase the conductivity by electrochemical reduction of the nanotubes. We observed that in acidic or alkaline electrolytes the nanotubes (NTs) are destroyed during the reduction process (Fig. 5a). This phenomenon occurs even in neutral solutions at reaction times of more than 1 minute. The reduced NTs were used as a substrate for silicon deposition. Figure 5b shows that the coating is mainly on the surface, leading to the assumption that the tubes are too close to each other (15-50 nm according to SEM analysis) for the IL-based electrolyte to reach the bottom of the substrate. Recently, Nemaga et al [62] discovered an electrolyte with hydrofluoric acid that provides homogeneous tubes with large spacing (100-200 nm). However, due to the potential hazards of HF, it would be advantageous to use HF-free electrolytes for large-scale production of this substrate. This is the subject of ongoing research.
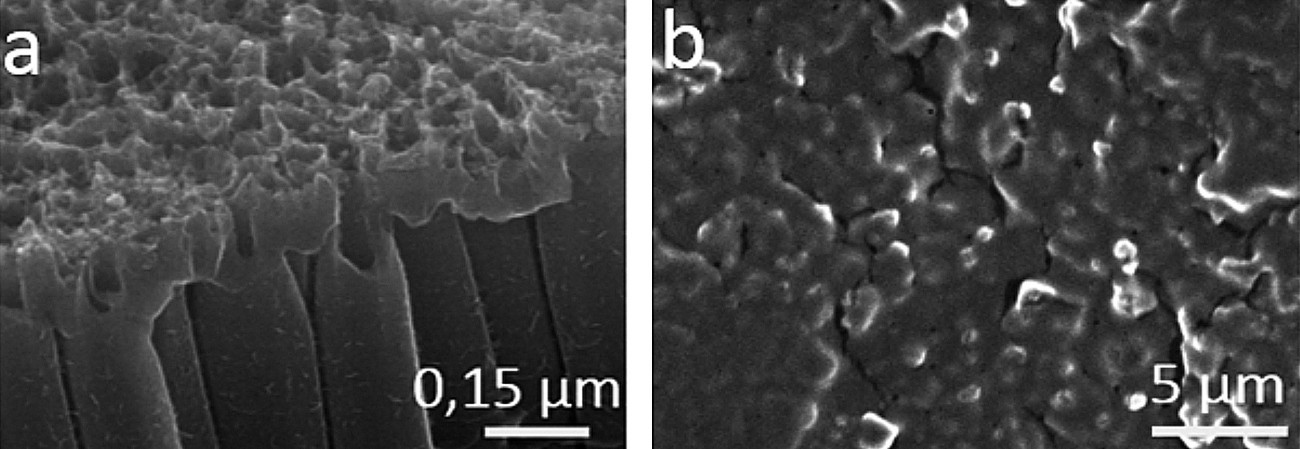 Fig. 5: SEM images of titanium foils anodized in glycerol with 2 wt% H2Oand 0.5 wt% NH4Fat 40 V - 75 min. Electrochemically reduced TiO2-NTs in KOH electrolyte (a) and silicon-coated NTs (b)
Fig. 5: SEM images of titanium foils anodized in glycerol with 2 wt% H2Oand 0.5 wt% NH4Fat 40 V - 75 min. Electrochemically reduced TiO2-NTs in KOH electrolyte (a) and silicon-coated NTs (b)
Recycling of electrolytes
![Abb. 6: Messung des elektrochemischen Stabilitätsfensters des frischen und des recycelten [BMP][TFSI]. Der Einschub zeigt eine detailliertere Ansicht des Stroms (ν = 10 mV s-1) Abb. 6: Messung des elektrochemischen Stabilitätsfensters des frischen und des recycelten [BMP][TFSI]. Der Einschub zeigt eine detailliertere Ansicht des Stroms (ν = 10 mV s-1)](/images/stories/Abo-2022-07/thumbnails/thumb_gt-2022-07-0037.jpg) Fig. 6: Measurement of the electrochemical stability window of the fresh and recycled [BMP][TFSI]. The inset shows a more detailed view of the current (ν = 10 mV s-1) The removal of the remaining precursor and the purification of the electrolyte from decomposition products is relatively simple. To check the purification, spectroscopic measurements can be carried out and the electrochemical stability window can be checked (Fig. 6).
Fig. 6: Measurement of the electrochemical stability window of the fresh and recycled [BMP][TFSI]. The inset shows a more detailed view of the current (ν = 10 mV s-1) The removal of the remaining precursor and the purification of the electrolyte from decomposition products is relatively simple. To check the purification, spectroscopic measurements can be carried out and the electrochemical stability window can be checked (Fig. 6).
As can be clearly seen, no cathodic peak can be observed in the recycled IL, whereas this is not the case with the fresh solution. However, the oxidation current increases at lower anodic voltages, which is probably due to small residual amounts of decomposition products in the IL. It also has a slightly yellow color, which may be due to these products [52]. In fact, it is known that color changes can result from impurities that are even below the detection limit of NMR spectroscopy [63]. However, the influence of coloration on spectroscopic methods with light absorption or emission makes it impossible to check the purity of ILs with these methods [64]. Therefore, a solution decolorization method based on the work of Earle and coworkers is used to verify the purity of recycled electrolytes using optical spectroscopic methods in addition to electrochemical techniques [64]. In summary, the absence of additional peaks, particularly in the cathodic region of the voltammetric data for the recycled IL, indicates that the IL no longer contains any electroactive species that would compromise its continued use as an electrolyte for silicon deposition. Therefore, the applied purification process is a suitable method for the recycling of spent electrolytes.
Summary
The electrochemical deposition of silicon from two different electrolytes was investigated. The different substrates showed similar behavior on the LSVs in PC. The deposition on the nickel substrates generally occurred at more negative potentials. The chronoamperograms showed higher currents for the copper substrates. However, despite the lower viscosity of the PC-based solution, the current for nickel is lower than in the IL electrolyte. This could be due to the decomposition of the electrolyte. The SEM analysis shows rough silicon layers. The layers obtained from IL are dense with small cracks and consist of small spherical objects. EDX confirms the presence of entrapped electrolyte molecules or their decomposition products. The deposition of silicon from PC leads to a superimposed structure with voids and holes. The EDX analysis confirms a high level of contamination with carbon and oxygen, which is due in particular to the decomposition of the electrolyte during deposition. An Ag/AgCl reference electrode for organic electrolytes provided a stable potential for the experiments over weeks (PC) up to five months (IL). Electrochemical deposition of silicon on TiO2 nanotubes was not possible due to the small tube spacing. SEM analyses showed that long reduction times and strongly acidic or alkaline media destroy the nanotubes. Purifying the spent electrolyte by a simple extraction method offers a simple and fast way to recycle the expensive solution. Although the color changed slightly yellowish, electrochemical measurements confirmed a thorough cleaning.
Acknowledgments
The authors gratefully acknowledge the financial support of the Cusanuswerk and the DFG project "In-situ spectroscopic investigations of high energy Li-S batteries based on new carbon cathodes" (ISIBAT) (project no.: 273723695).
Notes:
Read the article first published in English in 2019 under the title "Electrochemical deposition of silicon from organic electrolytes" at: www.jept.de
Further findings on silicon deposition at: www.db-thueringen.de
Literature
[1] El Abedin, S.Z.; Borissenko, N., Endres, F.: Electrodeposition of nanoscale silicon in a room temperature ionic liquid, Electrochemistry Communications, 6(5), (2004), 510-514
[2] Tsuyuki, Y.; Takai, H.; Fukunaka, Y.; Homma, T.: Formation of Si thin films by electrodeposition in ionic liquids for solar cell applications, Japanese Journal of Applied Physics, 57(8S3), (2018), 08RB11
[3] Mallet, J.; Molinari, M.; Martineau, F.; Delavoie, F.; Fricoteaux, P.; Troyon, M.: Growth of silicon nanowires of controlled diameters by electrodeposition in ionic liquid at room temperature, Nano letters, 8(10), (2008), 3468-3474
[4] Borisenko, N.; Zein El Abedin, S.; Endres, F.: In situ STM investigation of gold reconstruction and of silicon electrodeposition on Au (111) in the room temperature ionic liquid 1-butyl-1-methylpyrrolidinium bis (trifluoromethylsulfonyl) imide, The Journal of Physical Chemistry B, 110(12), (2006), 6250-6256
[5] Agrawal, A.K.; Austin, A.E.: Electrodeposition of silicon from solutions of silicon halides in aprotic solvents, Journal of The Electrochemical Society, 128(11), (1981), 2292-2296
[6] Gobet, J.; Tannenberger, H.. Electrodeposition of silicon from a nonaqueous solvent, Journal of The Electrochemical Society, 135(1), (1988), 109-112
[7] Zhao, G.; Meng, Y.; Zhang, N.; Sun, K.: Electrodeposited Si film with excellent stability and high rate performance for lithium-ion battery anodes, Materials Letters, 76, (2012) 55-58
[8] Munisamy, T.; Bard, A.J.: Electrodeposition of Si from organic solvents and studies related to initial stages of Si growth; Electrochimica Acta, 55(11), (2010), 3797-3803
[9] Bechelany, M.; Elias, J.; Brodard, P.; Michler, J.; Philippe, L.: Electrodeposition of amorphous silicon in non-oxygenated organic solvent, Thin Solid Films, 520(6), (2012), 1895-1901
[10] Vichery, C.; Le Nader, V.; Frantz, C.; Zhang, Y.; Michler, J.; Philippe, L.: Stabilization mechanism of electrodeposited silicon thin films, Physical Chemistry Chemical Physics, 16(40), (2014), 22222-22228
[11] Momma, T.; Aoki, S.; Nara, H.; Yokoshima, T.; Osaka, T.: Electrodeposited novel highly durable SiOC composite anode for Li battery above several thousands of cycles, Electrochemistry Communications, 13(9), (2011), 969-972
[12] Lv, R.; Yang, J.; Wang, J.; NuLi, Y.: Electrodeposited porous-microspheres Li-Si films as negative electrodes in lithium-ion batteries, Journal of Power Sources, 196(8), (2011), 3868-3873
[13] Epur, R.; Ramanathan, M.; Beck, F.R.; Manivannan, A.; Kumta, P.N.: Electrodeposition of amorphous silicon anode for lithium ion batteries, Materials Science and Engineering: B, 177(14), (2012), 1157-1162
[14] Gattu, B.; Epur, R.; Shanti, P.M.; Jampani, P.H.; Kuruba, R.; Datta, M.K.; Manivannan, A.; Kumta, P.N.: Pulsed current electrodeposition of silicon thin films anodes for lithium ion battery applications, Inorganics, 5(2), (2017), 27
[15] Nishimura, Y.; Fukunaka, Y.: Electrochemical reduction of silicon chloride in a non-aqueous solvent. Electrochimica Acta, 53(1), (2007), 111-116
[16] Schmuck, M.; Balducci, A.; Rupp, B.; Kern, W.; Passerini, S.; Winter, M.: Alloying of electrodeposited silicon with lithium-a principal study of applicability as anode material for lithium ion batteries, Journal of solid state electrochemistry, 14(12), (2010), 2203-2207
[17] Chen, X.; Gerasopoulos, K.; Guo, J.; Brown, A.; Wang, C.; Ghodssi, R.; Culver, J.N.: A patterned 3D silicon anode fabricated by electrodeposition on a virus-structured current collector, Advanced Functional Materials, 21(2), (2011), 380-387
[18] Hang, T.; Mukoyama, D.; Nara, H.; Yokoshima, T.; Momma, T.; Li, M.; Osaka, T.: Electrochemical impedance analysis of electrodeposited Si-O-C composite thick film on Cu microcones-arrayed current collector for lithium ion battery anode, Journal of Power Sources, 256, (2014), 226-232
[19] Bieber, A.L.; Massot, L.; Gibilaro, M.; Cassayre, L.; Taxil, P.; Chamelot, P.: Silicon electrodeposition in molten fluorides. Electrochimica Acta, 62, (2012), 282-289
[20] Zaykov, Y.P.; Zhuk, S.I.; Isakov, A.V.; Grishenkova, O.V.; Isaev, V.A.: Electrochemical nucleation and growth of silicon in the KF-KCl-K 2 SiF 6 melt, Journal of Solid State Electrochemistry, 19(5), (2015), 1341-1345
[21] Martineau, F.; Namur, K.; Mallet, J.; Delavoie, F.; Endres, F.; Troyon, M.; Molinari, M.: Electrodeposition at room temperature of amorphous silicon and germanium nanowires in ionic liquid. In IOP Conference Series: Materials Science and Engineering, IOP Publishing, Vol. 6, No. 1, (2009) 012012
[22] Liu, X.; Zhang, Y.; Ge, D.; Zhao, J.; Li, Y.; Endres, F.: Three-dimensionally ordered macroporous silicon films made by electrodeposition from an ionic liquid; Physical Chemistry Chemical Physics, 14(15), (2012), 5100-5105
[23] Park, J.; Kwon, K.; Kim, H.; Lee, C.K.: Electrodeposition of silicon from 1-butyl-3-methyl-pyridinium bis (trifluromethylsulfonyl) imide ionic liquid, Int. J. Electrochem. Sci, 8, (2013), 4206-4214
[24] Vlaic, C.A.; Ivanov, S.; Peipmann, R.; Eisenhardt, A.; Himmerlich, M.; Krischok, S.; Bund, A.: Electrochemical lithiation of thin silicon based layers potentiostatically deposited from ionic liquid, Electrochimica Acta, 168, (2015), 403-413
[25] Zhang, J.; Chen, S.; Zhang, H.; Zhang, S.; Yao, X.; Shi, Z.: Electrodeposition of crystalline silicon directly from silicon tetrachloride in ionic liquid at low temperature, Rsc Advances, 6(15), (2016), 12061-12067
[26] Shah, N.K.; Pati, R.K.; Ray, A.; Mukhopadhyay, I.: Electrodeposition of Si from an ionic liquid bath at room temperature in the presence of water, Langmuir, 33(7), (2017), 1599-1604
[27] Komadina, J.; Akiyoshi, T.; Ishibashi, Y.; Fukunaka, Y.; Homma, T.: Electrochemical quartz crystal microbalance study of Si electrodeposition in ionic liquid, Electrochimica Acta, 100, (2013), 236-241
[28] Nishimura, Y.; Fukunaka, Y.; Nishida, T.; Nohira, T.; Hagiwara, R.: Electrodeposition of Si thin film in a hydrophobic room-temperature molten salt, Electrochemical and Solid-State Letters, 11(9), (2008), D75-D79
[29] Pulletikurthi, G.; Lahiri, A.; Carstens, T.; Borisenko, N.; El Abedin, S.Z.; Endres, F.: Electrodeposition of silicon from three different ionic liquids: possible influence of the anion on the deposition process, Journal of Solid State Electrochemistry, 17(11), (2013), 2823-2832
[30] Tsuyuki, Y.; Fujimura, T.; Kunimoto, M.; Fukunaka, Y.; Pianetta, P.; Homma, T.: Analysis of Cathodic Reaction Process of SiCl4 during Si Electrodeposition in Ionic Liquids, Journal of The Electrochemical Society, 164(14), (2017), D994-D998
[31] Thomas, S.; Kowalski, D.; Molinari, M.; Mallet, J.: Role of electrochemical process parameters on the electrodeposition of silicon from 1-butyl-1-methylpyrrolidinium bis (trifluoromethanesulfonyl) imide ionic liquid, Electrochimica Acta, 265, (2018), 166-174
[32] Zhang, W.J.: A review of the electrochemical performance of alloy anodes for lithium-ion batteries, Journal of Power Sources, 196(1), (2011), 13-24
[33] Obrovac, M.N.; Chevrier, V.L.: Alloy negative electrodes for Li-ion batteries, Chemical reviews, 114(23), (2014), 11444-11502
[34] Etacheri, V.; Geiger, U.; Gofer, Y.; Roberts, G.A.; Stefan, I.C.; Fasching, R.; Aurbach, D.: Exceptional electrochemical performance of Si-nanowires in 1, 3-dioxolane solutions: a surface chemical investigation, Langmuir, 28(14), (2012), 6175-6184
[35] Rehnlund, D.; Lindgren, F.; Böhme, S.; Nordh, T.; Zou, Y.; Pettersson, J.; Bexell, U.; Boman, M.; Edström, K.; Nyholm, L.: Lithium trapping in alloy forming electrodes and current collectors for lithium based batteries, Energy & Environmental Science, 10(6), (2017), 1350-1357
[36] Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.G.: Lithium metal anodes for rechargeable batteries, Energy & Environmental Science, 7(2), (2014), 513-537
[37] Liu, Y.; Li, B.; Liu, J.; Li, S.; Yang, S.: Pre-planted nucleation seeds for rechargeable metallic lithium anodes, Journal of Materials Chemistry A, 5(35), (2017), 18862-18869
[38] Manthiram, A.: An outlook on lithium ion battery technology, ACS central science, 3(10), (2017), 1063-1069
[39] Kowalski, D.; Mallet, J.; Thomas, S.; Nemaga, A.W.; Michel, J.; Guery, C.; Molinary, M.; Morcrette, M.: Electrochemical synthesis of 1D core-shell Si/TiO2 nanotubes for lithium ion batteries, Journal of Power Sources, 361, (2017), 243-248
[40] Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J.: Silicon-based nanomaterials for lithium-ion batteries: a review, Advanced Energy Materials, 4(1), (2014), 1300882
[41] Macak, J.M.; Schmuki, P.: Anodic growth of selforganized anodic TiO2 nanotubes in viscous electrolytes, Electrochimica Acta, 52(3), (2006), 1258-1264
[42] Bauer, S.; Kleber, S.; Schmuki, P.: TiO2 nanotubes: Tailoring the geometry in H3PO4/HF electrolytes, Electrochemistry Communications, 8(8), (2006), 1321-1325
[43] Ng, S.W.; Yam, F.K.; Beh, K.P.; Hassan, Z.: Titanium dioxide nanotubes in chloride based electrolyte: an alternative to fluoride based electrolyte, Sains Malaysiana, 43(6), (2014), 947-951
[44] Brumbarov, J.; Kunze-Liebhäuser, J.: Silicon on conductive self-organized TiO2 nanotubes-A high capacity anode material for Li-ion batteries, Journal of Power Sources, 258, (2014), 129-133
[45] Ivanov, S.; Grieseler, R.; Cheng, L.; Schaaf, P.; Bund, A.: Electrochemical lithiation of Si modified TiO2 nanotube arrays, investigated in ionic liquid electrolyte, Journal of Electroanalytical Chemistry, 731, (2014), 6-13
[46] Cui, L.F.; Ruffo, R.; Chan, C.K.; Peng, H.; Cui, Y.: Crystalline-amorphous core- shell silicon nanowires for high capacity and high current battery electrodes, Nano letters, 9(1), (2008), 491-495
[47] Datta, M.K.; Maranchi, J.; Chung, S.J.; Epur, R.; Kadakia, K.; Jampani, P.; Kumta, P.N.:. Amorphous silicon-carbon based nano-scale thin film anode materials for lithium ion batteries, Electrochimica Acta, 56(13), (2011), 4717-4723
[48] Nguyen, C.C.; Song, S.W.: Interfacial structural stabilization on amorphous silicon anode for improved cycling performance in lithium-ion batteries. Electrochimica Acta, 55(8), (2010), 3026-3033
[49] Li, C.; Liu, C.; Wang, W.; Mutlu, Z.; Bell, J.; Ahmed, K.; Ye, R.; Ozkan, M.; Ozkan, C.S.: Silicon derived from glass bottles as anode materials for lithium ion full cell batteries, Scientific Reports, 7(1), (2017), 917
[50] Li, X.; Gu, M.; Hu, S.; Kennard, R.; Yan, P.; Chen, X.; Wang, C.; Sailor, M.J.; Zhang, J.-G.; Liu, J.: Mesoporous silicon sponge as an anti-pulverization structure for high-performance lithium-ion battery anodes, Nature communications, 5, (2014), 4105
[51] Hernandez, C.R.; Etiemble, A.; Douillard, T.; Mazouzi, D.; Karkar, Z.; Maire, E.; Guyomard, D.; Lestriez, B.; Roué, L.: A Facile and Very Effective Method to Enhance the Mechanical Strength and the Cyclability of Si-Based Electrodes for Li-Ion Battery, Advanced Energy Materials, 8(6), (2018), 1701787
[52] Clare, B.; Sirwardana, A.; MacFarlane, D.R.: Synthesis, purification and characterization of ionic liquids, In Ionic Liquids, Springer, Berlin, Heidelberg (2009), 1-40
[53] Seddon, K.R.; Stark, A.; Torres, M.J.: Influence of chloride, water, and organic solvents on the physical properties of ionic liquids, Pure and Applied Chemistry, 72(12), (2000), 2275-2287
[54] Mai, N.L.; Ahn, K.; Koo, Y.M.: Methods for recovery of ionic liquids-a review. Process Biochemistry, 49(5), (2014), 872-881
[55] Saheb, A.; Janata, J.; Josowicz, M.: Reference electrode for ionic liquids. Electroanalysis: An International Journal Devoted to Fundamental and Practical Aspects of Electroanalysis, 18(4), (2006), 405-409
[56] Bazito, F.F.; Kawano, Y.; Torresi, R.M.: Synthesis and characterization of two ionic liquids with emphasis on their chemical stability towards metallic lithium, Electrochimica Acta, 52(23), (2007), 6427-6437
[57] Armstrong, N.R.; Quinn, R.K.; Vanderborgh, N.E.: Heterogeneous charge transfer rates of the ferrocene oxidation in sulfolane, J. Electrochem. Soc., United States, 123(5) (1976)
[58] Zara, A.J.; Machado, S.S.; Bulhões, L.O.S; Benedetti, A.V.; Rabockai, T.: The electrochemistry of ferrocene in non-aqueous solvents, Journal of electroanalytical chemistry and interfacial electrochemistry, 221(1-2), (1987), 165-174
[59] Bond, A.M.; Oldham, K.;B., Snook, G.A.: Use of the ferrocene oxidation process to provide both reference electrode potential calibration and a simple measurement (via semiintegration) of the uncompensated resistance in cyclic voltammetric studies in high-resistance organic solvents, Analytical chemistry, 72(15), (2000), 3492-3496
[60] Tachikawa, N.; Katayama, Y.; Miura, T.: Electrode kinetics of some iron complexes in an imide-type room-temperature ionic liquid, Journal of The Electrochemical Society, 154(11), (2007), F211-F216
[61] Tsierkezos, N.G.: Cyclic voltammetric studies of ferrocene in nonaqueous solvents in the temperature range from 248.15 to 298.15 K, Journal of Solution Chemistry, 36(3), (2007), 289-302
[62] Nemaga, A.W.; Mallet, J.; Michel, J.; Guery, C.; Molinari, M.; Morcrette, M.: All electrochemical process for synthesis of Si coating on TiO 2 nanotubes as durable negative electrode material for lithium ion batteries, Journal of Power Sources, 393, (2018), 43-53
[63] Nockemann, P.; Binnemans, K.; Driesen, K.: Purification of imidazolium ionic liquids for spectroscopic applications, Chemical physics letters, 415(1-3), (2005), 131-136
[64] Earle, M.J.; Gordon, C.M.; Plechkova, N.V.; Seddon, K.R.; Welton, T.: Decolorization of ionic liquids for spectroscopy, Analytical chemistry, 79(2), (2007), 758-764


