- Part 2 - Theodor Freiherr von Grotthuß - continued from "Galvanotechnik" 8/2025
Baron Theodor von Grotthuß' discoveries in the field of electroplating are no less important than those of his contemporary Johann Wilhelm Ritter (see issue 8/2025). Nevertheless, Grotthuss' findings were only rediscovered and appreciated in the 20th century.
The von Grotthuß family is one of the oldest noble families in Germany. They emerged from the mists of millennia into the light of history in the 10th century in the area around Münster. At that time, they were still known by the Latin name of their ancestral seat, de Magna Domo.
A later document from 1269 names something like the progenitor of the family, Theodoricus de magna domo. Finally, in 1370, the Prince-Bishop of Münster names a descendant in a feudal charter, this time no longer in Latin but in German: a certain Stephanus receives Grotenhaus as a fief. This Magna Domo, the large house or Grothus in Westphalian, was located near the municipality of Nordkirchen, which today belongs to the district of Coesfeld.
The family grew and prospered, expanding its sphere of influence first to Hanover, then to Brandenburg and the Baltic Sea. With the Teutonic Order, the Grothusens finally arrived in the Baltic, by which time the name had already become Groitueß. On October 17, 1620, the family is enrolled as the von Grotthuß family in the 1st class of the Courland knighthood. In Geddutz, today Gedučiai (Lithuania), an offshoot of the dynasty owns extensive estates with a manor on them.
Theodor von Grotthuß, the subject of this essay, came from this offshoot and was named after the family patriarch Theodoricus more than 500 years later. In keeping with his rank, he naturally had other first names, so that his full and correct name was Christian Johann Dietrich Theodor Freiherr von Grotthuß, surnamed Theodor. He was born on January 20, 1785 in Leipzig, where his parents had just been traveling. They stayed with a friend of the family, Christoph Felix Weiße. He was a writer and teacher, an important representative of the Enlightenment and a renowned rococo poet. Above all, however, he is regarded by literary scholars as the founder of children's and youth literature. Conveniently, Weiße became the godfather of little Theodor, and in this capacity Weiße enrolled his godson as a student at Leipzig University as a child.
Enrolled at the university as a child
Theodor's parents are the estate owners Dietrich Ewald and Elisabeth Eleonore. Both are very artistic, Dietrich composes and Elisabeth sings excellently. As was customary in upper-class circles at the time, Theodor was educated by tutors and trained in all the important subjects. Although there were already village schools, it was socially unacceptable in those days to be educated with the children of ordinary people.
Of course, he learned French at an early age, the language of the upper classes at the time. But, as his parents and tutors had not actually intended, he had little interest in artistic subjects such as poetry, painting and music. He threw himself vehemently into the natural sciences. Even as a boy, he was particularly taken with experimental chemistry. This passion would stay with him for the rest of his life.
In 1803, at the age of 18, he moved away from Geddutz. He took up his godfather's option and began studying natural sciences in Leipzig. But he did not stay in Leipzig for long. Just six months later, he moved to Paris. His family's means (and his language lessons) made it possible for him to enrol at the École Polytechnique, which was already renowned at the time and where the scientific giants of the time taught. Among others, Grotthuss was inspired by the proponents of the antiphlogiston theory. Claude-Louis Berthollet and his colleague Antoine François de Fourcroy considered the phlogiston theory to be nonsense and tried to refute it with all their might.
The phlogiston theory was introduced to science in the 17th century by the German alchemist, chemist and physician Georg Friedrich Stahl. Stahl assumed that when a body is heated, it absorbs the (naturally hypothetical) substance "phlogiston", but releases it again during combustion. The role of oxygen in combustion was not yet known at the time. The phlogiston theory was important in the interpretation of reduction and oxidation processes and the different potentials of various compounds (in modern terms, their redox potential). In the 1770s, the first attempts to refute this theory appeared and people began to describe and quantify oxygen (which had only recently been discovered) in combustion processes in more detail. Since the discovery of oxygen, the phlogiston theory has therefore been counted among the scientific errors and seen as an outdated scientific paradigm of its time. But Grotthuß was still in the middle of the scientific debate about the mysterious substance in 1803.
Phlogiston theory - a scientific fallacy
Unfortunately, there were already reasons to leave Paris again in 1804. The political situation between France and Russia came to a head. Geddutz, Grotthuss' home town, was in Courland, which was a Russian governorate at the time. And so Theodor Grotthuß moved on to Rome.
But this was to be decisive for him. In Rome, he came into contact with Pacchiani's experiments on the electrolytic decomposition of water. As early as 1805, he went public with his own theory on the decomposition of liquids by galvanic currents, which made his name famous at a stroke. The treatise, which appeared in Rome, was published in international scientific journals over the next few years, including in his home region of Courland [1]. This was followed by a brief detour to Naples, where he also enrolled at the university.
 A house in the village of Žeimelis, Lithuania. It bears a street sign with the name T. Grotuso. This is how the Lithuanians keep the scientist's name alive
A house in the village of Žeimelis, Lithuania. It bears a street sign with the name T. Grotuso. This is how the Lithuanians keep the scientist's name alive
In 1806, Theodor also breaks up his tents in Italy. He had to return home, his duties as heir to Grotthuss's large agricultural business were calling him. But he takes a detour via Paris, where he stays for a few more months. His essay "Sur la décomposition de l'eau et des corps qu'elle tient en dissolution à l'aide de l'électricité galvanique" (The decomposition of water and the bodies dissolved in it by galvanic electricity) [2] was published there in the same year. In it Grotthuß describes: "If a current of galvanic electricity is allowed to flow through a saturated metal salt solution, the intensity of which is proportional to the distance covered by the liquid between the ends of the conducting wires, strange phenomena are observed which are interesting even to those who do not wish to delve into their causes. Oxygen develops at the end of the wire connected to the zinc disk, while metal is reduced at the wire connected to the copper disk, assuming a symmetrical arrangement that extends in the direction of the galvanic current."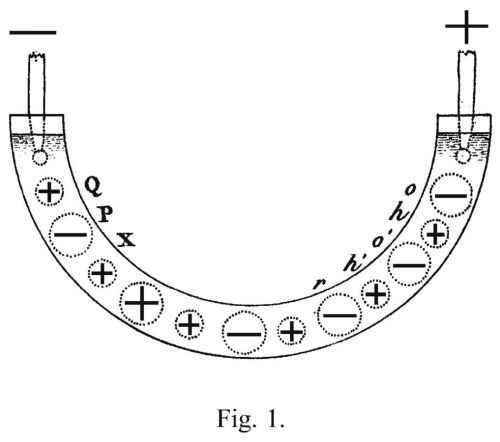 Schematic representation of the Grotthuss mechanism by the scientist himself. Reprinted in the publication "The decomposition of water and the bodies dissolved in it by galvanic electricity"
Schematic representation of the Grotthuss mechanism by the scientist himself. Reprinted in the publication "The decomposition of water and the bodies dissolved in it by galvanic electricity"
As Grotthuss experimented with many metals, he also noticed that these behaved differently after the current was applied. In the same essay [2] he writes: "Not all dissolved metals are decomposed by galvanic electricity. From manganese nitrate I obtained gas bubbles at the negative pole, instead of a galvanic precipitate; it seems that if under the same circumstances the dissolved metal has more affinity for oxygen than hydrogen, the water alone is decomposed." His basic idea to explain the current mechanism was therefore that a polarity is formed between the components of water, hydrogen and oxygen, when current flows, the water particles therefore align themselves in a polar arrangement and when hydrogen or oxygen is released at the electrodes, a regrouping takes place between neighboring components that continues throughout the entire liquid. This idea made the spatially separated development of hydrogen and oxygen plausible, could be transferred to other media and explained why positively or negatively charged particles do not accumulate at the electrodes [1].
When Grotthuß returned to his home in Courland around 1807, he immediately had to devote himself to the concerns of the estate. His scientific research had reached a critical point. He has little time, no almost inexhaustible library at his disposal and is completely on his own. All the more intensely he throws himself into experiments. In the process, he comes across a chemical process in aqueous solutions that is named after him, the Grotthuss mechanism. In such a solution, the positive charges of the oxonium ions can be transferred very quickly. They do not have to diffuse through the solution like other ions, but the positive charge is simply passed on to another water molecule. Wikipedia writes today: "This mechanism is a chain mechanism: instead of transporting protons through the solution, bonds and hydrogen bonds are broken and re-formed. This makes it possible to "flip" the bonds and pass on the charge very quickly [3].
Grotthuss mechanism in solutions
However, Grotthuß is also mistaken in crucial respects, perhaps because he lacks direct contact with other scientists. For example, he assumes that water consists of one atom of oxygen and one atom of hydrogen. If a current is applied, the oxygen atom would become the positive pole, while the hydrogen atom would become the negative pole - and this idea is clearly wrong, as modern chemistry knows today.
If you compare the lives of the two great galvanic scientists of the Romantic period, Johann Wilhelm Ritter and Theodor von Grotthuß, you will notice both far-reaching similarities and a major discrepancy: Both personalities dealt successfully and productively with galvanism, with wet chemistry. Like Ritter, Grotthuß also researched the nature of light and color, inspired by Goethe. Ritter was desperately poor, while Grotthuß was able to fall back on a large fortune from his aristocratic family. Ritter was a bird of paradise who moved in the highest intellectual circles with Goethe, Humboldt, Hardenberg and Schelling. Grotthuss, on the other hand, after his studies in Leipzig, Paris and Rome (which Ritter could never have afforded), experimented alone in the remote Russian governorate of Courland in the Baltic States.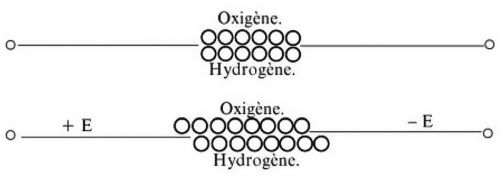 Schematic representation of water by Grotthuss himself. The diagram shows the chemist's erroneous assumption that water consisted of one atom of oxygen and one atom of hydrogen. After a current is applied, oxygen would become the positive pole and hydrogen the negative pole
Schematic representation of water by Grotthuss himself. The diagram shows the chemist's erroneous assumption that water consisted of one atom of oxygen and one atom of hydrogen. After a current is applied, oxygen would become the positive pole and hydrogen the negative pole
While Ritter's research results were known and remained known, Grotthuß' results were forgotten almost immediately after their publication. Grete Ronge writes in the Neue Deutsche Biographie: "The theories (by Grotthuß, editor's note) were so readily and naturally accepted that the author was forgotten and had to assert priority rights just 15 years later." In 1819 Grotthuß published his treatises on the chemical effectiveness of light. In this publication, he formulates and establishes the photochemical absorption law. This was a quarter of a century before Draper, yet the law is called Grotthuß-Draper.
Unfortunately, both men, Ritter and Grotthuß, also died early. In all probability, Ritter died of consumption at the age of 34. Grotthuß ended his own life on March 26, 1822. He was 37 years old and suffered from an incurable illness.
Literature
[1]: Ronge, Grete: "Grotthuß, Theodor Freiherr von"; Neue Deutsche Biographie 7 (1966), pp. 171-172 [online version]; URL: https://www.deutsche-biographie.de/pnd119546965.html#ndbcontent
[2]: C.J.T. de Grotthuss: Sur la décomposition de l'eau et des corps qu'elle tient en dissolution à l'aide de l'électricité galvanique, Ann. Chim. (Paris), 58 (1806), pp. 54-73 Grotthuß
[3]: https://de.wikipedia.org/wiki/Grotthu%C3%9F-Mechanismus
The illustrations are from Wikimedia Commons




