RoKKa was a publicly funded project that ran for around three years and ended in October 2024. As part of the project, measurement programs were launched and test setups set up at the municipal wastewater treatment plant in Erbach, Württemberg. The focus was on demonstrating a wastewater treatment plant that has been converted into a biorefinery, which extracts and processes raw materials from wastewater, including phosphorus, nitrogen andCO2 in concentrated form by means of sludge digestion. The topic is doubly interesting for electroplating technology: on the one hand, because phosphorus is needed for Ni-P coatings and phosphating, for example, and on the other, because it is recovered electrochemically.
Finite raw material phosphorus
The world's population is growing steadily, resulting in an increasing demand for food. To meet this demand, artificial fertilizers containing phosphorus are used in agriculture. Their production goes hand in hand with the extraction of mineral rock phosphates. Phosphorus cannot be substituted at this point. Different scenarios describe that the global phosphorus reserves that can be mined will be exhausted in 150 to 300 years [1]. A study by the International Fertilizer Association (IFA) from 2023 states that reserves will be exhausted within the next 222 to 458 years [2]. Germany has no natural sources of phosphorus that are suitable for economic extraction, which is why recycling the raw material appears attractive.
Obligation to recover phosphorus
In Germany, phosphorus recovery is becoming mandatory. Following an amendment to the Sewage Sludge Ordinance (AbfKlärV) in 2017, sewage treatment plant operators with a capacity of 100,000 population equivalents (PE) or more will be legally obliged to recover phosphorus from 2029. From 2032, this obligation will also apply to wastewater treatment plants with an expansion size of 50,000 PE and more [3]. In future, wastewater treatment plants with the aforementioned expansion stages will have to recover phosphorus if the dry mass of their sewage sludge contains at least 20 grams of phosphorus per kilogram of sewage sludge [3].
Application
Particularly at sewage treatment plants with biological phosphorus elimination (Bio-P), comparatively high phosphorus concentrations occur in the sludge water that is produced during sewage sludge dewatering after sludge digestion. In order to extract phosphorus directly from a liquid phase, the Fraunhofer Institute for Interfacial Engineering and Biotechnology (IGB) has developed and patented an electrochemical process. With ePhos, phosphate and ammonium present in water can be precipitated to form struvite and crystallized. The precipitation process completely dispenses with the addition of liquid chemicals and electrochemically doses the required magnesium in pure form. The product struvite is also known as magnesium ammonium phosphate hexahydrate (Mg (NH4) [PO4]*6 H2O) and can be used directly as a fertilizer in agriculture. Other conceivable applications for the ePhos process are the treatment of liquid fermentation residues - for example from biogas plants - or the treatment of phosphorus-containing waste water from the food industry.
Functional principle
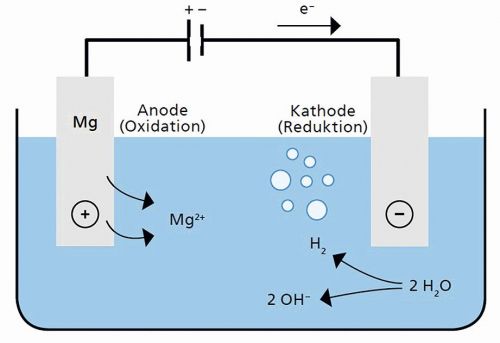
The basic principle of the ePhos process is based on classic electrolysis. Figure 1 shows the processes that take place during P-salt precipitation: A pair of electrodes is immersed in an aqueous liquid containing phosphate and ammonium and energized. The anode consists of pure magnesium and serves as a sacrificial anode. It releases Mg2+ ions, which initially dissolve in the liquid. Ammonium (NH4+), phosphate (PO43-) and water combine with the dissolved magnesium to form struvite. Wastewater from sewage sludge dewatering usually has a pH value close to 7. Struvite forms in this environment, but initially remains in solution without raising the pH value. The ePhos process therefore utilizes the property of the inert cathode to split water molecules. On the one hand, this produces molecular hydrogen (H2), which outgasses from the liquid. On the other hand, hydroxide ions (OH-) are formed, which raises the pH value of the water. This shifts the solubility curve of the liquid, which means that initially less and less struvite can be held in solution as the pH value increases. As a result, the phosphorus salt crystallizes as a solid. After precipitation, the phosphorus salt can be recovered from the liquid using a suitable separation technique - such as sedimentation or filtration.
In the past, the ePhos technology has already been piloted several times at municipal wastewater treatment plants. In situ, 80 to 90 % of the incoming phosphorus could be eliminated in continuous operation over a period of months. Depending on the plant configuration and the phosphorus concentration in the water, the energy requirement for electrolysis was between 0.5 and 3.7 kWh per cubic meter of sludge water treated. The struvite obtained was separated from the wastewater and dried. Figure 2 shows a sample obtained during pilot testing.
Technology development
The pilot plant was further developed on the basis of the experience gained in the previous pilots. The geometry of the drop shaft reactor, which was used for the first time during the pilot in the RoKKa project, represents a significant improvement. In addition, the footprint was to be reduced by using a belt filter instead of a sedimentation tank for crystal separation from the liquid.
 Fig. 3: ePhos pilot plant
Fig. 3: ePhos pilot plant
Figure 3 shows the current reactor geometry of the ePhos pilot plant. The design consists of a plastic housing mounted on an aluminum frame. There is an electrolysis cell to the left and right of the mixing tank. The water is fed from the outside through a reaction gap in which the electrolysis takes place. In the photo, the places where the drop shafts with the sacrificial anodes made of magnesium are located are marked with a red area. A blue field indicates the area where the inert cathode is installed inside the cell. After the phosphate-containing water enters the mixing tank through the reaction gap under the drop shafts, the struvite crystals can form and grow there. They are then separated from the liquid.
 Fig. 4: Detailed view of the drop shaft
Fig. 4: Detailed view of the drop shaft
Figure 4 shows a detailed view of a drop shaft reactor. Ingots of pure magnesium are used as sacrificial anodes. As the anodes are consumed, a reactor geometry had to be developed that allows the electrodes to be replaced with little maintenance effort. Fresh ingots can be added from above by opening the drop shaft; the anodes are contacted from above with a contact plunger that follows the height level of the ingots, depending on the consumption level.
Structure within the RoKKa project
Although phosphorus salt precipitation also involves the recovery of nitrogen in the form of ammonium, it only marginally reduces the nitrogen concentrations in the wastewater. For this reason, a nitrogen recovery module was installed downstream of the ePhos pilot plant as part of the RoKKa pilot project: a pilot plant for the AmmoRe process developed at the Fraunhofer IGB. The process is used to recover sulphuric acid ammonium sulphate from nitrogen-containing wastewater for fertilizer production. During the pilot phase, the nitrous oxide emissions generated in the aeration tank of the wastewater treatment plant were measured. Nitrous oxide is one of the climate-relevant gases produced at sewage treatment plants. The pilot project showed that around 10 % of nitrous oxide emissions can be avoided through nitrogen recovery [4]. Another pilot module was used to captureCO2, which is contained in the digester gas. The focus here was not only on reducing greenhouse gas emissions, but also on using theCO2 as a raw material for new products. For example, a microalgae reactor was gassed with it andCO2 was also converted into formic acidusing electrosynthesis. In order to demonstrate the closing of small, internal cycles, the microalgae were also fed with the phosphorus salts produced at the sewage treatment plant. Part of the pilot project was also the establishment of biological phosphorus elimination (Bio-P), which had not previously been used at the Erbach wastewater treatment plant. This was expected to result in an increased phosphorus load in the filtrate water flow, which was to be recovered with ePhos.
Pilot module ePhos
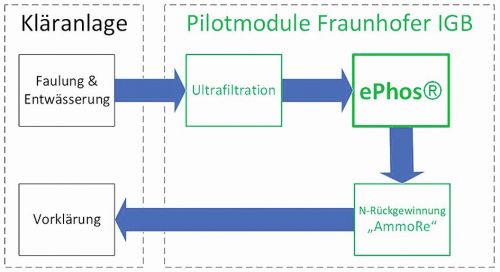 Fig. 5: ePhos process control in the RoKKa projectFig.5 shows the process control of the nutrient recovery modules that were set up for the RoKKa project at the wastewater treatment plant in Erbach. The digested sludge is dewatered using a chamber filter press and the filtrate water is stored in an elevated tank. Before the water is transported to the ePhos plant, it passes through an ultrafiltration process. The main purpose of this process step is to protect the membranes of the AmmoRe module from suspended particles. The ePhos module extracts P salts from the phosphorus-rich water, which are then separated from the liquid phase using the belt filter. As the water is then still rich in nitrogen, the nitrogen recovery AmmoRe is connected downstream of the ePhos module to produce nitrogen fertilizer. The depleted filtrate water is then returned to the inlet of the wastewater treatment plant. The two technologies therefore not only have the task of producing recyclates for use in agriculture, but also of significantly reducing the return load of phosphorus and nitrogen in the filtrate water flow.
Fig. 5: ePhos process control in the RoKKa projectFig.5 shows the process control of the nutrient recovery modules that were set up for the RoKKa project at the wastewater treatment plant in Erbach. The digested sludge is dewatered using a chamber filter press and the filtrate water is stored in an elevated tank. Before the water is transported to the ePhos plant, it passes through an ultrafiltration process. The main purpose of this process step is to protect the membranes of the AmmoRe module from suspended particles. The ePhos module extracts P salts from the phosphorus-rich water, which are then separated from the liquid phase using the belt filter. As the water is then still rich in nitrogen, the nitrogen recovery AmmoRe is connected downstream of the ePhos module to produce nitrogen fertilizer. The depleted filtrate water is then returned to the inlet of the wastewater treatment plant. The two technologies therefore not only have the task of producing recyclates for use in agriculture, but also of significantly reducing the return load of phosphorus and nitrogen in the filtrate water flow.
Results
Figure 6 shows the results of various operating phases of the ePhos plant. The graph shows the input and output concentrations of phosphorus in the water, as well as the resulting case efficiency. The average retention time of the water in the system during the four operating phases is also shown. Struvite was already precipitated with ePhos on this scale in previous pilot projects. In RoKKa, it was also possible to extract struvite from the wastewater using ePhos. However, in the initial phase (phase 1), only approx. 30 % of the incoming phosphorus could be extracted from the water. It was found that the water contained much less phosphorus (approx. 10 mg/L) than initially assumed. One reason for this was that the phosphorus load in the filtrate water flow did not increase during the project period despite the piloted Bio-P process. In addition, the already low phosphorus concentration was further reduced by the ultrafiltration. It was therefore decided in phase 2 to add additional phosphorus, which increased the case efficiency from 30 to 40 %. In the further course of phase 2, the retention time was increased by throttling the inflow. In addition, ultrafiltration was installed downstream of the ePhos system in order to avoid an additional reduction in the phosphorus load. In phase 3, the retention time was increased again in order to determine the maximum possible case efficiency. In some cases, efficiencies close to 70 and 80 % were achieved, but these showed a wide variation. In phase 4, the aim was to achieve a uniform input phosphorus concentration of approx. 30 mg/L with a retention time of 3.7 hours. By the end of the pilot phase, case efficiencies close to 60 % were achieved throughout.
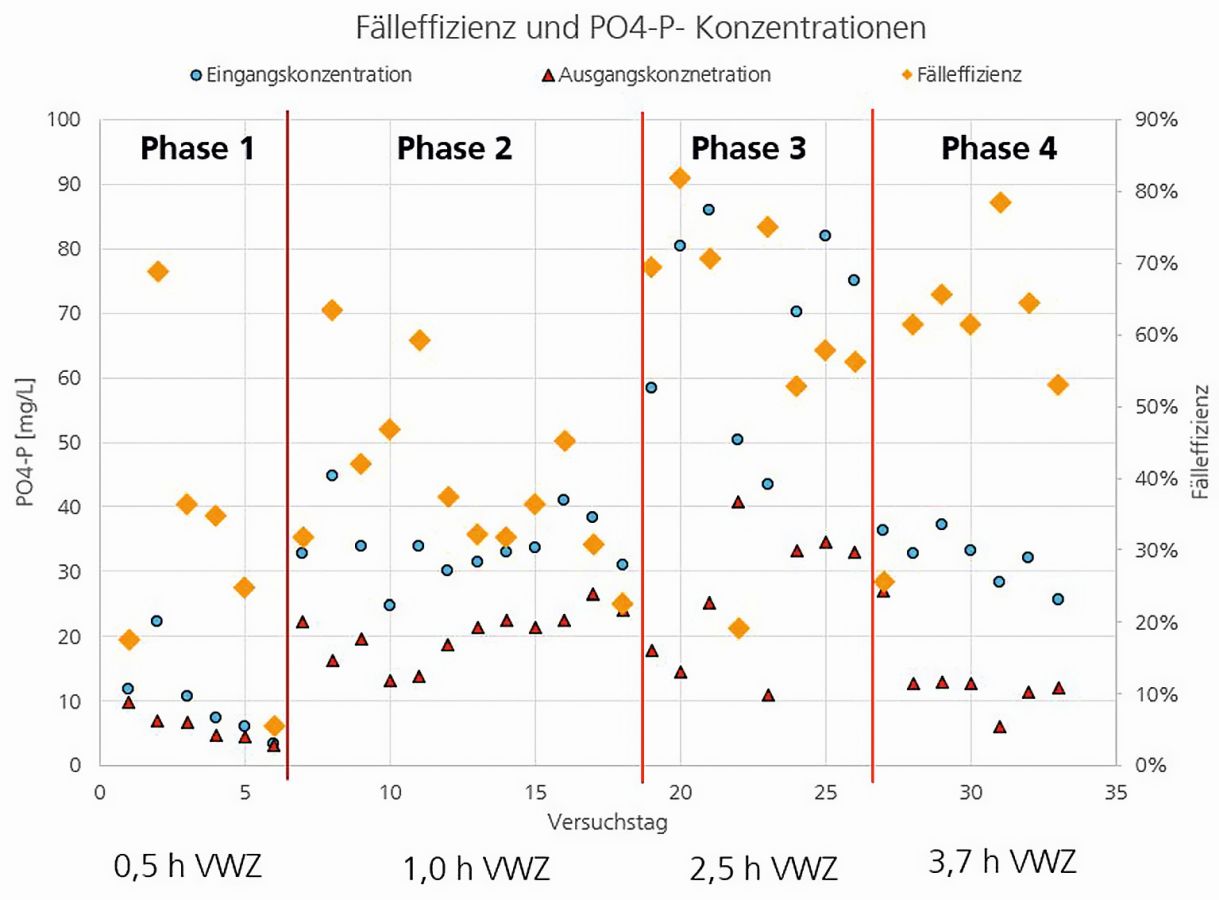 Fig. 6: Illustration of phosphate concentration and case efficiency over the course of different operating phases of ePhos in the RoKKa project
Fig. 6: Illustration of phosphate concentration and case efficiency over the course of different operating phases of ePhos in the RoKKa project
Important findings
The most important finding of the pilot project was the significance of the reaction volume of the ePhos system. The testing of a belt filter for separating the P salts instead of a sedimentation tank reduced the system volume considerably. This change was the main difference to previous pilots and explains the unusually low case efficiencies, as the retention times were considerably reduced. Despite all this, the belt filter proved to be an extremely suitable unit for separating struvite from the wastewater, as Figure 7 shows.
Another important finding came from the analysis of the product. During pilot testing in Erbach, a P-salt mixture was produced that had a high proportion of calcium phosphates. This is due to the water hardness of the water in Erbach at >20 °dH, which indicates a high input of calcium ions. An extremely positive finding was that all heavy metal concentrations in the product were below the limit values stipulated in the Fertilizer Ordinance. Equally positive was the fact that the new reactor design of the drop shaft was able to prove itself in operation.
 Fig. 7: Separation of P salts at the belt filter
Fig. 7: Separation of P salts at the belt filter
The pilot project has once again shown that phosphate-containing fertilizers can be easily extracted from filtrate water streams from the sludge digestion of municipal wastewater treatment plants using ePhos. The plant concept is now being further developed using the findings from the RoKKa pilot plant. The focus here is primarily on the choice of separation process for the P salts and the associated dimensioning of the ideal system volume. The aim is to achieve a long-term case efficiency of over 90 % again with future systems.
The RoKKa project (raw material source sewage sludge and climate protection at sewage treatment plants) was funded by the Baden-Württemberg Ministry for the Environment, Climate Protection and the Energy Sector and the EU via the ERDF BioAbCycling program.
The article is based on a presentation at the Ulmer Gespräch 2025 on May 14/15 in Ulm
Additional information from the editorial team
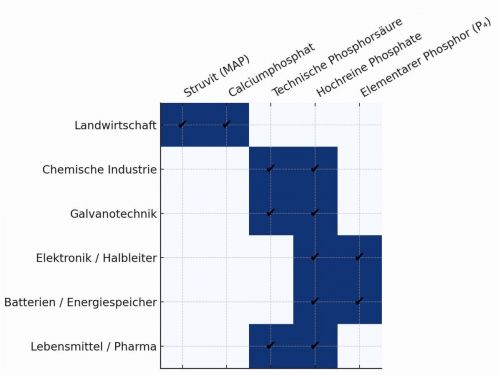
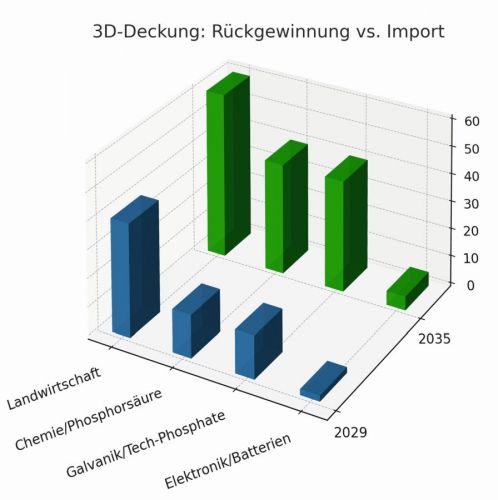
Industrial demand for phosphorus and projections for reducing import dependency
Sources: Analysis by AI using sources from the Federal Environment Agency, ScienceDirect, Phosphorus Platform and Fertilizers Europe
Literature
[1] Sustainable phosphorus supply, Issue Brief No. 39, Sonja Kind, April 2020, Office of Technology Assessment at the German Bundestag (TAB), p. 1
[2] Phosphate Rock, Resources & Reserves, April 2023, Prepared for: International Fertilizer Association, Argusmedia, p.7
[3] German Bundestag printed matter 18/10884, 18th legislative period 18.01.2017, Ordinance of the Federal Government, Ordinance on the Reorganization of Sewage Sludge Utilization, p. 102
[4] Final brochure 2024, RoKKa - The update for the sewage treatment plant, Malte Thormann Tobias Morck, p.19