Aggressive high-temperature corrosion, metal dusting, occurs in reducing atmospheres, such as those found in hydrogen production plants. As process conditions are becoming more and more extreme in order to achieve higher efficiencies, the targeted development of materials with higher metal dusting resistance for structural system components is necessary. Coatings also play a role here.
Alloy developers are faced with the challenge of incorporating high proportions of oxide formers into the material. Copper can be increasingly used as a further alloying element in the future, as it is considered to inhibit catalytic attack. This opens up further options for innovative alloy development.
What is metal dusting?
The issue of high-temperature corrosion on structural metals is often not recognized as a modern topic. Traditionally, metal dusting is mainly known from the chemical and petrochemical industry, e.g. from processes such as pyrolysis, steam cracking, steam reforming, partial oxidation, the production of ammonia, urea and nitric acid, coking plants and the production and processing of synthesis gas. The atmospheres typical of these plants lead to a particularly aggressive form of high-temperature corrosion, known as "metal dusting". Metal dusting occurs in environments with temperatures between approx. 400 and 900 °C with a maximum around 600 °C and often leads to spontaneous component damage. Although many processes such as the production of coke take place at temperatures above the critical range of metal dusting attack, the lower temperatures in the downstream periphery (downstream area) lead to localized damage there. Typically, the process atmospheres of such processes consist of a high CO and H2 content in addition to other molecules such asCO2, N2, alcohols and water vapor, so the processes are associated with high material requirements, e.g. for metallic reactors and pipes.
![Abb. 1: Metallische Proben vor und nach einer Auslagerung unter Metal-Dusting-Bedingungen. a) Monel Alloy 400 zeigt einen flächigen Angriff [1], und bei b) wird der Angriff der HR-235-Legierung in Form von Pit-Bildung sichtbar [2]](/images/stories/Abo-2023-06/thumbnails/thumb_gt-2023-06-041.jpg) Fig. 1: Metallic samples before and after aging under metal dusting conditions. a) Monel Alloy 400 shows a surface attack [1], and in b) the attack of the HR-235 alloy is visible in the form of pit formation [2]
Fig. 1: Metallic samples before and after aging under metal dusting conditions. a) Monel Alloy 400 shows a surface attack [1], and in b) the attack of the HR-235 alloy is visible in the form of pit formation [2]
One of the biggest challenges for the industry is that metal dusting often only occurs after an incubation period that is difficult to predict [3, 4]. The metal dusting attack describes the process in which metallic materials are attacked from the surface by the incorporation of carbon into the material. The corrosion product is black (Fig. 1a) and is called "coke". It consists of fine metal particles, graphite, amorphous carbon and fine oxide particles [5]. All common base elements for high-temperature alloys iron (Fe), nickel (Ni) and cobalt (Co) are - under given conditions - susceptible to metal dusting and even catalyze the attack. In most cases, the attack occurs locally in so-called "pits" (Fig. 1b). Three reactions are decisive for the separation of C from the gas phase.
Synthesis gas reaction: CO(g) + H2(g)→C(s) + H2O(g) <1>
Boudouard reaction: 2 CO(g) → C(s) +CO2(g)<2>
Methane cleavage: CH4(g) → C(s) + 2H 2(g)<3>
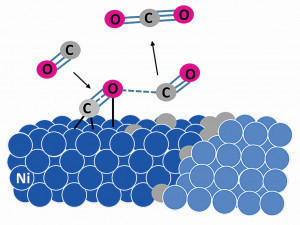 Fig. 2: Schematic representation of the metal dusting mechanism from the decomposition of the carbon-containing molecule (here CO) to the diffusion of the carbon on the metal surface and the accumulation at a grain boundary
Fig. 2: Schematic representation of the metal dusting mechanism from the decomposition of the carbon-containing molecule (here CO) to the diffusion of the carbon on the metal surface and the accumulation at a grain boundary
4 steps describe the mechanism (Fig. 2) [6]:
- C-containing gases (e.g. CO or CxH2x+2) adsorb and decompose catalytically on the alloy surface.
- The bond between C and the metal surface is weak, so the C atoms can diffuse into the metal matrix along easy diffusion paths (cracks, edges, grain boundaries). In nickel-based alloys, C is dissolved in the material and carbide precipitates (typically chromium carbides) are formed.
- As soon as C accumulates in the material, the graphite crystallizes in the area of lattice defects such as grain and phase boundaries.
- The increase in volume due to graphite formation causes strong stresses, causing the metallic material to be blasted out from the inside.
Why is metal dusting relevant for the energy transition?
Due to the energy crisis, processes for hydrogen production (H2) and storage have experienced a new upswing. The often reducing, carbon-rich atmospheres in combination with high temperatures create a whole new relevance for the high-temperature corrosion form of metal dusting, especially in the context of hydrogen production. According to Kalis' color theory of hydrogen [7], the following starting materials and production processes are affected by metal dusting:
- High-temperature electrolysis (green H2)
- Methane pyrolysis (turquoise H2)
- Biomass gasification (yellow H2)
- Steam reforming withCO2 capture and storage (CCS) and combinations of fossil fuels with CCS (blue H2)
- Steam reforming, partial oxidation, autothermal reforming from natural gas without CCS (gray H2)
- Coal gasification from lignite (brown H2)
- Coal gasification from hard coal (black H2).
It is clear that most hydrogen production processes are affected by metal dusting. This applies both to processes that use fossil fuels and those that are based on renewable sources.
Currently, around 95 % of hydrogen worldwide is produced by steam reforming (SMR process), whereby fossil gas, mainly natural gas, is burned to CO and H2 (synthesis gas) [8]. The corresponding chemical reaction of synthesis gas production from methane is:
CH4(g) + H2O(g) → CO(g) + 3H2(g)
Processes that are being considered for the production of green H2 and where there is a risk of material failure due to metal dusting are high-temperature electrolysis and steam reforming from regeneratively produced alcohols [9, 10]. In high-temperature co-electrolysis, synthesis gas (H2 and CO) is produced using water,CO2 and electricity. The aim of steam reforming from alcohols is to produce the alcohols used from biomass in a regenerative way.
Why is metal dusting relevant to our everyday lives?
Hydrogen and thus its production is of crucial importance due to its potential as an energy carrier of the future, but also as a reactant for many processes in the chemical and petrochemical industry, whose products we come into contact with every day (e.g. fertilizers, cosmetics, pharmaceuticals, polymers, dyes, detergents, fine chemicals). The Haber-Bosch process, which went down in history as "bread from air" and is used to synthesize ammonia, is also dependent on the supply of hydrogen. In addition to processes for hydrogen production, CO (synthesis gas production) orCO2 (oxidation of CO) is also required as a raw material in many processes. The production processes for these gaseous raw materials are the same as those listed above for the production of H2 alone. Accordingly, these processes also lead to material failure due to metal dusting.
According to a recent study by DECHEMA e. V., assuming the best-case scenario, at least 87% of fossil fuels will still be required for nitric acid production (Ostwald process) and 92% for urea production (urea) in 2030 [11]. Since the production of urea, for example, requiresCO2 as well as H2, the aim in this area is not necessarily to operate the process withoutproducing CO2. Rather, innovative process management should be used to utilize theCO2 produced during synthesis gas production in educt synthesis, thus making the process climate-neutral. This shows that the use of fossil fuels is still expedient in some areas ifCO2 can be reused directly by adapting the process control. Which is why atmospheres that cause metal dusting are also preserved in these processes.
Trends in alloy development for metal dusting environments
In order to make processes more and more efficient, process conditions are becoming increasingly aggressive (faster temperature changes, more aggressive atmospheres, higher pressures) and are therefore an increasing challenge for the stability of the materials used against high-temperature corrosion. Therefore, in times of energy transition, the relevance of this attack, which is still not fully understood for structural materials, is enormous and will not lose importance in the future. The targeted development of metal-dusting-resistant materials for new applications, as well as more resistant materials to conserve resources and prevent material failure, will become even more attractive in the future. There are two approaches to alloy development:
- Alloying with oxide formers that form an oxide layer as a barrier between the aggressive atmosphere and the metallic substrate (compare Fig. 3 a).
- Alloying with elements that catalytically inhibit the dissociation of the carbon-containing molecules (shown schematically in Fig. 3 b).
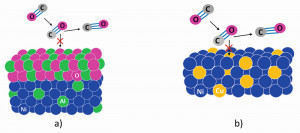 Fig. 3: Schematic representation of both alloying concepts to increase metal-dusting resistance: a) an oxidic barrier and b) alloying with copper to inhibit carbon deposition
Fig. 3: Schematic representation of both alloying concepts to increase metal-dusting resistance: a) an oxidic barrier and b) alloying with copper to inhibit carbon deposition
Alloying with oxide formers
In processes with metal-dusting atmospheres, Fe- and Ni-based alloys with high Al and Cr contents are typically used. These materials usually form protective Cr2O3, Cr2MnO4 or Al2O3 layers. If the protective oxide layer fails locally and it is not possible to form a new one, the component is attacked at this point by very rapidly growing component damage in the form of pits. It is important that the oxide-forming elements form protective, stable oxide layers even at low oxygen partial pressure - metal dusting takes place in reducing environments with p(O2) < 10-15 Pa.
As the exact mechanism of metal dusting is not fully understood, the factors influencing it are not yet completely clear and alloy selection and further development are associated with a certain degree of uncertainty. For example, it is unclear what effect the exact gas composition and process pressure have [12, 13]. As a rule of thumb, the proportion of oxide formers and in particular chromium in the alloy can be used as a measure of the alloy's resistance to metal dusting; the higher the Cr content, the better the resistance. However, very high levels of oxide formers have a negative effect on the workability and machinability of the alloy, e.g. brittleness increases. The formation of brittle phases also increases with increasing Cr content, while notched impact strength and weldability decrease. In order to achieve a balance in composition between corrosion resistance and mechanical properties, it is important to be able to determine the exact proportions of oxide formers (and other elements that influence the formation of the protective oxide layer) that lead to the resistance of the material. Since 1972, there have been various approaches to calculating a so-called Cr equivalent (Cräqu) for this purpose:
- Cräq = Cr (in % by weight) + 2 -Si (in % by weight) > 22 % by weight [14]
- Cräq = Cr (in % by weight) + 2 -Si (in % by weight) > 24 % by weight [15]
- Cräq = Cr (in % by weight) + 3 - (Si + Al) (in % by weight) >
24 wt.-% [16] - Cr > 28 wt.% in Ni-based alloys independent of Al (high Al content reduces carbon accumulation on the surface) [17]
- Cr (in wt.%) + Al (in wt.%) > 33 wt.% [18, 19]
- Low Fe content in Ni alloy [20,21]
- Cr ≥ 29.5 wt%, Al ≥ 2.0 wt% Al, Fe = 2.7-4.2 wt% (variations in Cr content between 28.9-29.4 wt% have a major influence) [22].
This list suggests that the complexity of such an estimation is far greater than can be represented by a simple summation of the weighted proportions of the typical oxide formers (Al, Si, Cr). Elements such as iron or manganese also have an influence on the resistance of the material.
Alloying with inhibiting elements
Elements such as tin (Sn), copper (Cu) and germanium (Ge) have been proven to reduce the metal-dusting attack. The theory is that these elements have a catalytically inhibiting or at least neutral effect on the adhesion and dissociation of carbon-containing molecules (see Fig. 3b) [23-25]. Since phase boundaries form an easy diffusion path, it is important to avoid their formation; the formation of intermetallic phases and the resulting phase boundaries increase the susceptibility to metal-dusting attacks [26-29]. Therefore, Ge and Sn are applied to the surface by coatings [30-32]. Due to the high solubility of copper in the nickel matrix, Cu is the only one of the three elements mentioned that is considered as an alloying element to improve metal-dusting resistance.
In previous studies, it was hypothesized that a Cu content of at least 10 wt% (in stainless steel) [33] or 20 wt% in binary Ni-Cu alloys leads to a complete inhibition of metal-dusting attack [34, 35]. This has recently been disproved in experiments on binary, single-phase (face-centered cubic carbon) Ni-Cu alloys [36]. Corresponding images showing the carbon attack can be seen in Figure 4. Even if the exact limit value and its influence by other alloying elements is still unclear, it has been proven that Cu contents of around 10 wt% lead to a considerable improvement in metal-dusting resistance.
![Abb. 4: Makrobilder (links) und entsprechende Querschliffe (rechts) der Werkstoffe Monel Alloy 400 und der Modelllegierung Ni-32Cu während einer Auslagerung bei 620 °C in 20 % CO – 20 % H2 – 1 % H2O – 8 % CO2 – 51 % Ar bei 18 bar für 961 h [36] gt 2023 06 046](/images/stories/Abo-2023-06/thumbnails/thumb_gt-2023-06-041.jpg) Fig. 4: Macro images (left) and corresponding cross-sections (right) of the materials Monel Alloy 400 and the model alloy Ni-32Cu during ageing at 620 °C in 20 % CO - 20 % H2 - 1 % H2O - 8 % CO2 - 51 % Ar at 18 bar for 961 h [36]
Fig. 4: Macro images (left) and corresponding cross-sections (right) of the materials Monel Alloy 400 and the model alloy Ni-32Cu during ageing at 620 °C in 20 % CO - 20 % H2 - 1 % H2O - 8 % CO2 - 51 % Ar at 18 bar for 961 h [36]
Attempts are already being made to utilize the effect of Cu with the latest alloys available on the market from various manufacturers: The nickel-based alloy NSSMC 696 from Nippon Steel contains 2.1 wt% Cu in addition to 30 wt% Cr, the alloy HR-235 from Haynes even contains 31 wt% Cr and 3.8 wt% Cu [37]. However, these alloys contain only small amounts of Cu and also high quantities of oxide formers. The Cu is also intended to increase protection in the event of oxide layer failure or at the beginning of an exposure, when a protective oxide layer has not yet formed. The focus of future alloy design is now on the combination of higher proportions of copper - or even another alloying element with an inhibiting effect - with high proportions of oxide formers, but without leading to increased precipitation of phases. Research and development in the industry will have to face this challenge in the future.
Literature
[1]Schmitt, F.: Investigations of the metal dusting resistance of additive manufactured Ni-based alloys as a function of surface treatments, Master's thesis, TH Bingen, Frankfurt, 2021
[2]Ulrich, A.S.; Schmitt, F.; Reiff, L.; Jahns, K.; Krupp, U.; Galetz, M.C.: The Effect of Surface Finish on Metal Dusting, lecture EUROCORR 2021, digital, 21.09.2021
[3]Natesan, K.; Zeng, Z.: Development of Materials Resistant to Metal Dusting Degradation, Argonne National Lab.(ANL), Argonne, IL (USA), Final Report, 2007
[4]Fabas, A.; Monceau, D.; Josse, C.; Lamesle, P.; Put, A.R.-V.: Mechanism of Metal Dusting Corrosion by Pitting of a Chromia-Forming Alloy at Atmospheric Pressure and Low Gas Velocity, Corros. Sci, 107, 2016, 204-210, https://doi.org/10.1016/j.corsci.2016.02.033
[5]Szakalos, P.; Lundberg, M.; Pettersson, R.: Metal Dusting on an Alumina Forming Ni-Base Alloy, Corros. Sci, 48 (7), 2006, 1679-1695, https://doi.org/10.1016/j.corsci.2005.05.023
[6]Young, D.J.; Zhang, J.; Geers, C.; Schütze, M.. Recent Advances in Understanding Metal Dusting: A Review, Mater. Corros, 62 (1), 2011, 7-28, https://doi.org/10.1002/maco.201005675
[7]Kalis, M.: Hydrogen - We need a color theory and a detection system for green hydrogen, https://www.erneuerbare-energien-hamburg.de/de/blog/details/wasserstoff-wir-brauchen-eine-farbenlehre-und-ein-nachweissystem-fuer-gruenen-wasserstoff.html, April 2023
[8]Brun, K.; Allison, T.C.: Machinery and Energy Systems for the Hydrogen Economy, Elsevier, 2022
[9]Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B.: Production of Hydrogen from Ethanol: Review of Reaction Mechanism and Catalyst Deactivation, Chem. Rev., 112 (7), 2012, 4094-4123, https://doi.org/10.1021/cr2000114
[10]Zurek, J.; Margaritis, N.; Naumenko, D.; Menzler, N.H.; Quadakkers, W.J.: Behavior of Metallic Materials in Simulated Service Environments of CO2/H2O Co-Electrolysis Systems for Power-to-X Application, Oxid. Met., 92, 2019, 353-377, https://doi.org/10.1007/s11085-019-09927-9
[11]Ausfelder, F.; Herrmann, E.O.; González, L.F.L.: Perspective Europe 2030: Technology Options for CO2-Emission Reduction of Hydrogen Feedstock in Ammonia Production, DECHEMA Gesellschaft für Chemische Technik und Biotechnologie e.V., 2022
[12]Madloch, S.; Dorcheh, A.S.; Galetz, M.C.: Effect of Pressure on Metal Dusting Initiation on Alloy 800H and Alloy 600 in CO-Rich Syngas, Oxid. Met. 2018, 89, 483-498, https://doi.org/10.1007/s11085-017-9801-x
[13]Schlereth, C.; Hack, K.; Galetz, M.C.: Parameters to Estimate the Metal Dusting Attack in Different Gases, Corros. Sci, 206, 2022, 110483, https://doi.org/10.1016/j.corsci.2022.110483.
[14]Schueler, R.C.: Metal Dusting. Hydrocarb. Process, 51 (8), 1972, 73
[15]Schillmoller, C.M.: Solving High-Temperature Problems in Oil Refineries and Petrochemical Plants, Solving High-Temp. Probl. Oil Refineries, Petrochem Plants, 93 (1), 1986, 83-87
[16]Parks, S.B.; Schillmoller, C.M.: Improve Alloy Selection for Ammonia Furnaces, Hydrocarb. Process, 76 (10), 1997
[17]Röhnert, D.; Schütze, M.; Weber, T.: Performance of Several Nickel Base Alloys in Metal Dusting Atmospheres, In CORROSION 2007, OnePetro, 2007
[18]Hermse, C.; van Wortel, H.: Applicability Of Coatings To Control Metal Dusting, OnePetro, 2009
[19]Hermse, C.G.M.; van Wortel, J.C.: Metal Dusting: Relationship between Alloy Composition and Degradation Rate, Corros. Eng. Sci. Technol., 44 (3), 2009, 182-185, https://doi.org/10.1179/174327809X419140
[20]Hattendorf, H.; Hermse, C.G.M.; IJzerman, R.M.: The Influence of Alloying Elements on Metal Dusting Behavior of Nickel Chromium Alloys and Their Statistical Correlation, Mater. Corros, 70 (8), 2019, 1385-1399, https://doi.org/10.1002/maco.201810593
[21]Li, B.; Gleeson, B.; Chen, W.-T.; Hattendorf, H.: Effects of Minor Alloying Elements on the Metal-Dusting Behavior of Ni-Based Alloys, In CORROSION 2020, OnePetro, 2020
[22]Schlereth, C.; Oskay, C.; Hattendorf, H.; Nowak, B.; Galetz, M.C.: Influence of Al and Fe Additions on Metal Dusting of NiCr Alloys, Mater. Corros, 73 (9), 2022, 1346-1358, https://doi.org/10.1002/maco.202112935.
[23]Oden, L.L.; Gokcen, N.A.: Sn-C and Al-Sn-C Phase Diagrams and Thermodynamic Properties of C in the Alloys: 1550 °C to 2300 °C, Metall. Trans. B, 24, 1993, 53-58
[24]Trimm, D.L.: Catalysts for the Control of Coking during Steam Reforming, Catal. Today, 49 (1-3), 1999, 3-10, https://doi.org/10.1016/S0920-5861(98)00401-5
[25]Nikolla, E.; Holewinski, A.; Schwank, J.; Linic, S.: Controlling Carbon Surface Chemistry by Alloying: Carbon Tolerant Reforming Catalyst, J. Am. Chem. Soc. 2006, 128 (35), 11354-11355, https://doi.org/10.1021/ja0638298
[26]Chun, C.M.; Mumford, J.D.; Ramanarayanan, T.A.: Carbon-Induced Corrosion of Nickel Anode, J. Electrochem. Soc. 2000, 147 (10), 3680, https://doi.org/3680. 10.1149/1.1393958
[27]Zhang, J.; Young, D.J.: Kinetics and Mechanisms of Nickel Metal Dusting I. Kinetics and Morphology, Corros. Sci. 2007, 49 (3), 1496-1512, https://doi.org/10.1016/j.corsci.2006.08.008
[28]Zhang, J.; Young, D. J.: Coking and Dusting of Fe-Ni Alloys in CO-H2-H2O Gas Mixtures, Oxid. Met., 70, 2008, 189-211, https://doi.org/10.1007/s11085-008-9115-0
[29]Galetz, M.C.; Schlereth, C.; White, E.M.: Behavior of Copper-Containing High-Entropy Alloys in Harsh Metal-Dusting Environments, Mater. Corros, 72 (7), 2021, 1232-1242, https://doi.org/10.1002/maco.202012075
[30]Geers, C.; Galetz, M.; Schütze, M.: Investigation of the Effect of the Alloy 600 Substrate for the Stability of a Ni3Sn2 Coating for Metal Dusting Protection at 620 °C, Surf. Coat. Technol., 215, 2013, 2-6, https://doi.org/10.1016/j.surfcoat.2012.04.100
[31]Madloch, S.; Galetz, M.C.; Geers, C.; Schütze, M.: Development of a Metal Dusting Resistant Functional Coating by Sn and Al Pack Cementation, Surf. Coat. Technol., 299, 2016, 29-36, https://doi.org/10.1016/j.surfcoat.2016.04.067
[32]Galetz, M.C.; Oskay, C.; Madloch, S.: Microstructural Degradation and Interdiffusion Behavior of NiAl and Ge-Modified NiAl Coatings Deposited on Alloy 602 CA, Surf. Coat. Technol., 2019, 364, 211-217, https://doi.org/10.1016/j.surfcoat.2019.02.048
[33]Zhang, J.; Young, D.J.: Effect of Copper on Metal Dusting of Austenitic Stainless Steels, Corros. Sci, 49 (3), 2007, 1450-1467, https://doi.org/10.1016/j.corsci.2006.06.032.
[34]Nishiyama, Y.; Moriguchi, K.; Otsuka, N.; Kudo, T.: Improving Metal Dusting Resistance of Transition-Metals and Ni-Cu Alloys, Mater. Corros, 56 (11), 2005, 806-813, https://doi.org/10.1002/maco.200503883
[35]Chun, C.; Desai, S.; Ramanarayanan, T.A.: Metal Dusting Resistant Copper-Based Materials, Corrosion, 68 (9), 2012, 810-821, https://doi.org/10.5006/0609
[36]Jahns, K.; Ulrich, A.S.; Schlereth, C.; Reiff, L.; Krupp, U.; Galetz, M.C.: The Effect of Cu Content and Surface Finish on the Metal Dusting Resistance of Additively Manufactured NiCu Alloys, Oxid. Met., 96, 2021, 241-256, https://doi.org/10.1007/s11085-021-10037-8
[37]Li, B.; Deodeshmukh, V.; Schlereth, C.; Ulrich, A.S.; Galetz, M.C.: Metal Dusting Resistance of N06235 Alloy and Its Weld Overlay Under High Pressure Condition, In AMPP Annual Conference+ Expo, OnePetro, 2022


