Silver is increasingly valued for contact coatings in the electromobility industry. The article explains the development and investigation of electrolytes for the deposition of silver coatings with increased wear resistance. The main investigation concerned a cyanide-containing hard silver electrolyte made of silver-antimony alloys with the addition of graphite in powder form as a lubricant. The influence of different process parameters was determined during the research. For comparison with the main electrolyte, tests were carried out with a cyanide-containing silver-bismuth electrolyte and a cyanide-free silver-antimony electrolyte, both with graphite.
Due to the high electrical conductivity of silver (61.39 -106 S/m) [1] and the low price compared to gold, the metal appears to be a promising option for coating connectors. However, silver coatings have various limitations. Silver reacts with the sulphur contained in the air to form silver sulphide (Ag2S) [2]. In addition, pure silver coatings recrystallize easily, especially at high temperatures, making the coatings softer [1]. The coatings also have lower abrasion resistance, reduced wear and higher insertion and withdrawal forces. In order to optimize the quality of the silver coatings, the measures mentioned in [1] were taken. The hardness can be increased by adding other metals or by adding certain chemical elements/substances. The improvement and stabilization of the sliding properties can be achieved by the co-deposition of lubricants in the silver matrix.
It was investigated how connector coatings can be improved by electrochemical deposition of hard silver-graphite layers. Graphite achieves friction coefficients of 0.12, depending on the humidity [3]. The deposited graphite improves the service life of the composite by reducing the coefficient of friction [4]. The suitability of silver graphite coatings for self-lubricating electrical contacts has been known for decades [5].
Review of hard silver electrolytes
Many suppliers of electroplating chemicals introduced various processes for the deposition of silver alloys that have a relatively high hardness, e.g. silver-antimony (AgSb), silver-palladium (AgPd) and silver-bismuth (AgBi) [6]. The addition of antimony to cyanide silver baths increases the hardness and gloss of the silver coatings. In order to keep the antimony in solution in the alkaline baths, it must be added as a complex compound [7]. In 1966, a patent was filed describing the preparation of cyanide "hard silver baths" with cadmium sulphoselenide and soluble antimony complex compounds. The use of such electrolytes was described as a way of achieving bright coatings with adjustable and permanent hardness [8].
Silver coatings with improved sliding properties due to graphite
Electrolytic deposition of dispersion layers was developed in the early 1960s and only found practical application a few years later [9]. The technique offers a good opportunity to combine the properties of different metallic and non-metallic materials in a specifically defined material [10].
As silver and graphite are insoluble in each other, the production technique of silver graphite layers was initially limited to powder metallurgy [4] [11]. As thin layers (< 20 µm) are difficult to produce using powder metallurgy, research was carried out into how these could be electroplated [12].
The Siemens company described the first composite metals made of silver graphite for electrical contacts in the German patent literature in 1909 [13].
Experimental electrolyte components / working conditions
Brass sheets were used for the experiments. The samples were pretreated in the following steps: Decoction degreasing, electrolytic degreasing, decapitation, nickel plating and pre-gold/silver plating. After sample pretreatment, the hard silver-graphite layer can be electrolytically applied to the samples. Soluble silver anodes were used for the experiments. Table 1 shows the electrolytes used.
|
Electrolyte component /working conditions |
Cyanide-containing AgSb+graphite |
Cyanide-containing AgBi+graphite |
Cyanide-free AgSb+graphite |
|
Arguna 630 preparation salt |
50 g/L |
||
|
Potassium cyanide 98-99 % |
130 g/L |
50 g/L |
|
|
Potassium silver cyanide 54 % |
30 g/L Ag |
40 g/L Ag |
|
|
Arguna 630 Brightener NF |
10 mL/L |
||
|
Arguna GAM additive 1 |
0.8 g/L Sb |
||
|
Umicore wetting agent 39 |
10 mL/L |
1 to 5 mL/L |
|
|
Potassium hydroxide, chem. pure |
35 g/L |
75 g |
|
|
Arguna 635 Preparation salt |
40 g/L |
||
|
Arguna 635 Brightener 1-1 |
10 mL/L |
||
|
Arguna 635 Brightener 2 |
5 mL/L |
||
|
Arguna 635 Additive HV 2 |
1.25 g/L Bi |
||
|
Argunas 3430 Preparation salt |
185 g/L |
||
|
Arguna 3430 preparation solution |
0.5 g/L Sb |
||
|
Umicore silver methanesulfonate solution |
20 g/L Ag |
||
|
Arguna C-100 graphite powder |
Addition of 10 to 60 g/L |
30 g/L |
30 to 40 g/L |
|
Arguna C-100 brightener additive 1 |
1 to 6 mL/L |
||
Layer characterization
Qualitative and quantitative analyses
The coating composition was determined by energy-dispersive X-ray fluorescence analysis (XRF) [14][15] using the Fischerscope XAN 250 from Helmut Fischer. Using optical glow discharge spectroscopy [16] [17], both a qualitative and quantitative analysis of the coating was carried out at the fem Research Institute. The graphite incorporation rate was also determined gravimetrically. This was followed by optical observation of the coating using the VHX 7100 digital microscope from Keyence [17], the VK X1000 confocal laser scanning microscope from Keyence [18] and the Phenom XL scanning electron microscope from Thermo Fisher [19].
Tribological investigation
The coefficients of friction (COF) and wear were investigated using the TRB3 tribometer from Anton Paar. A silver rivet with a spherical geometry and a diameter of 5.6 mm was mainly used as the counter body and a flat substrate (sample) as the base body. The test was carried out in linear mode over a friction length of 10 mm.
Hardness measurement
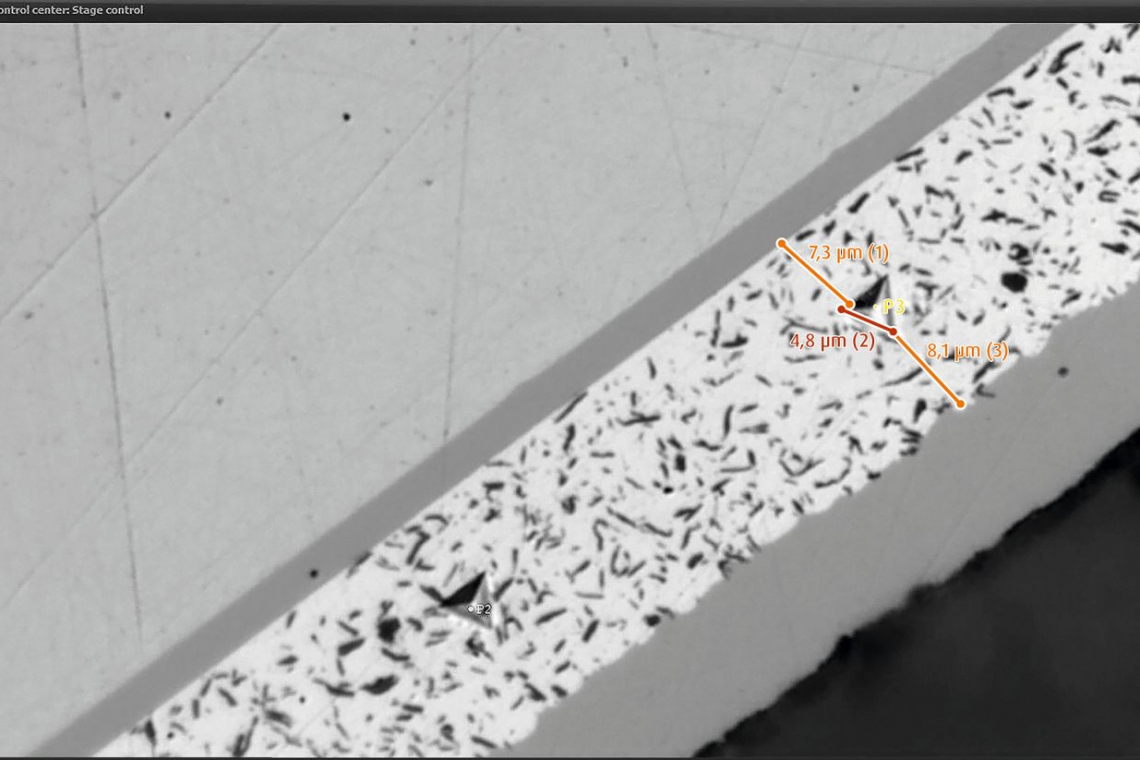
The hardness of the samples was determined using the UNAT nanoindenter from ASMEC Advanced Surface Mechanics with Berkovich indenter. A three-sided diamond pyramid (see cover picture), which is pressed into the test specimen with a force F, was used as an indenter [20]. The hardness values determined in this way were converted into Vickers hardness (unit: HV).
Results and discussion
After coating the samples with a hard silver graphite electrolyte based on Arguna 630, the coatings are optically matt. The composition of the hard silver layer shows that when the current density is increased from 1 to 6 A/dm2, the antimony content in the layer increases from approx. 0.8 % to approx. 2.6 %. These results only refer to the silver and antimony content. Graphite is not detected by XRF and was therefore subsequently determined both by glow discharge spectroscopy (GDOES) and gravimetrically. At a graphite content of 20 to 60 g/L in the electrolyte, the graphite content in the layer increases from 0.4 to 1.2 %. The samples were examined before and after a 336-hour heat treatment at 180 °C.
Sliding properties
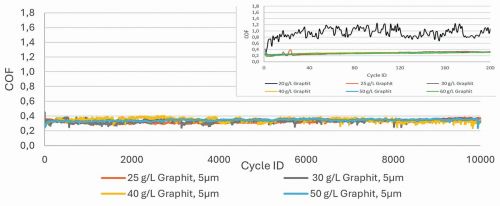
It was important to determine whether the addition of graphite to the hard silver electrolyte could achieve coefficients of friction comparable to those achieved with silver graphite (COF 0.2-0.4). As can be seen in Figure 2, there is a relatively large jump in the coefficient of friction downwards at 20 g/L graphite. From 25 g/L graphite, the coefficient of friction is within the desired range and the measurements are reproducible. The coatings were tested over 10,000 cycles (Fig. 1) and no significant increase in the coefficient of friction was observed until the end of the friction cycles. There is a nickel barrier layer (COF approx. 0.7 to 1) under the AgSb+graphite layer. If the layers had been rubbed through, the values would have risen after rubbing through. The AgBi+graphite layers were deposited from a cyanide electrolyte at a current density of 3 A/dm2. In the tribological tests, a shift in the coefficient of friction into the desired range was also observed with a graphite addition of 30 g/L (Fig. 3). The depositions with the cyanide-free electrolyte were carried out with a low current density of 1 A/dm2. A higher graphite content (40 g/L) was required to achieve friction coefficients < 0.4. One reason for this could be the lower working current density (Fig. 2).
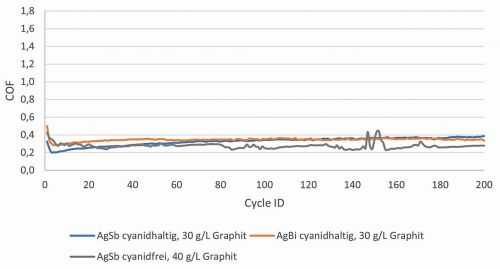 Fig. 2: Comparison of the friction coefficients of different hard silver coatings with graphite. Deposition parameters: Stirring speed: 300 rpm, temperature: 25 °C, test parameters: 2 N, Ag rivet
Fig. 2: Comparison of the friction coefficients of different hard silver coatings with graphite. Deposition parameters: Stirring speed: 300 rpm, temperature: 25 °C, test parameters: 2 N, Ag rivet
Hardness
The hardness of the deposited layers decreases with increasing graphite concentration in the electrolyte. The measured decrease in hardness is rather low in absolute terms in relation to the amount of graphite added. With regard to the antimony content, the hardness increases with increasing content of the element in the coating.
The hardness of the layers of cyanide-free AgSb+graphite and cyanide-containing AgBi+graphite electrolytes were also measured. It was found that coatings made from cyanide-free AgSb+ graphite electrolytes are significantly harder (Table 2).
|
Electrolyte |
Hardness without heat aging |
Hardness after heat aging (336 h; 180°C) |
|
AgSb+graphite (containing cyanide) |
140-150 HV |
95-125 HV |
|
AgSb+graphite (cyanide-free) |
170-180 HV |
120-130 HV |
|
AgBi+graphite (containing cyanide) |
130-150 HV |
125-130 HV |
Contact transition resistances
The co-deposition of elements with lower electrical conductivity than silver worsens the overall electrical conductivity of the deposited composite material. However, it is important to find a good compromise so that the layer has the longest possible service life.
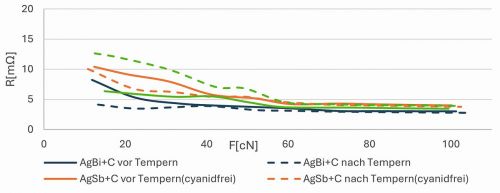 Fig. 3: Contact transition resistances of different hard silver layers with graphite. Test parameters: 0.1 to 1 A, Au rivet
Fig. 3: Contact transition resistances of different hard silver layers with graphite. Test parameters: 0.1 to 1 A, Au rivet
When examining the contact transition resistances, the coatings made of AgBi+graphite showed the lowest values, which improved even further after heat aging. In contrast to these samples, the samples from the cyanide-containing AgSb+graphite electrolyte exhibited higher contact transition resistances even before heat aging, which were even higher (>10 mΩ) after temperature exposure in the low force range (<60 cN) (Fig. 3). The cyanide-free AgSb+graphite layer exhibits the highest contact transition resistance before heat aging, but this decreases after heat aging as with AgBi+graphite. However, all contact transition resistances are within the desired range for the EV application (max. 10 mΩ from a contact force of 1 N). All samples have values of less than 5 mΩ from 60 cN.
Comparison with other silver coatings
Finally, layers of different silver electrolytes were deposited and compared in terms of sliding properties, hardness and contact transfer resistance. Fine silver (FS), hard silver, silver-graphite (AgC) and hard silver-graphite layers were deposited for this purpose. The tribological properties were investigated using the same parameters as mentioned above (see chapter Sliding properties). The desired sliding properties were only achieved with AgC and AgSb+graphite (Fig. 4). Hardness tests were then carried out, which showed that although hard silver graphite has a lower hardness than hard silver, it has a higher hardness than the other silver layers investigated (Table 3).
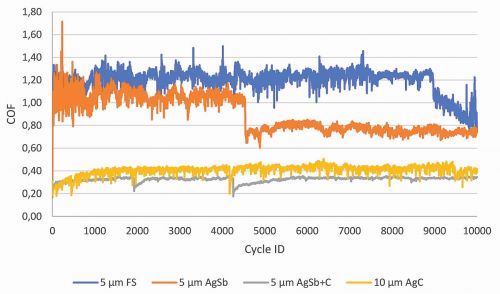 Fig. 4: Friction coefficients of different silver coatings. Deposition temperature: 25 °C, test parameters: 2 N, Ag rivet
Fig. 4: Friction coefficients of different silver coatings. Deposition temperature: 25 °C, test parameters: 2 N, Ag rivet
|
Fine silver |
Hard silver |
Silver graphite |
Hard silver with graphite (Sb content > 1 wt.%) |
|
|
Hardness range before heat aging |
approx. 80 HV |
170 - 200 HV |
75 - 85 HV |
150 - 160 HV |
|
Hardness range after heat aging |
120 - 150 HV |
- |
80 - 125 HV |
|
|
Coefficient of friction (COF) |
1 - 1,4 |
0,8 - 1,6 |
0,2 - 0,4 |
0,2 - 0,4 |
|
Graphite content per liter of electrolyte |
0 g/L |
0 g/L |
100 g/L |
From 25 g/L |
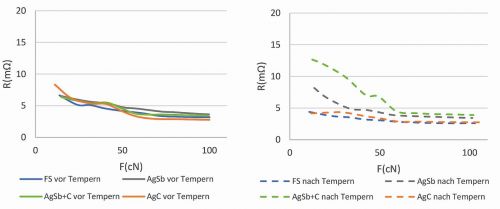 Fig. 5: Comparison of layers of fine silver, silver graphite, hard silver, hard silver with graphite before and after heat aging: deposition temperature: 25 °C, test parameters: 0.1 to 1 A, Au rivet
Fig. 5: Comparison of layers of fine silver, silver graphite, hard silver, hard silver with graphite before and after heat aging: deposition temperature: 25 °C, test parameters: 0.1 to 1 A, Au rivet
In contrast, fine silver has the lowest contact transition resistance of all coatings. Before heat aging, the coatings generally have comparable contact transition resistance values, but after temperature exposure, the results for fine silver and AgC coatings improve, while AgSb and AgSb+graphite coatings deteriorate (Fig. 5).
Wear analysis
For the tests, samples were coated with fine silver (FS), AgC, AgSb, and AgSb+graphite (AgSb+C). The tested coatings were tribologically tested against a solid silver rivet material (FS-VM) on the one hand and against the copper rivets coated with 5 µm fine silver, 15 µm AgC, 5 µm AgSb and 5 µm AgSb+C on the other. All tests were carried out over 10,000 cycles. The coefficients of friction of the AgSb+C layers are constant between 0.2 and 0.4 for all rivets, i.e. the AgSb+C layer on the sheet is not rubbed through (Fig. 6).
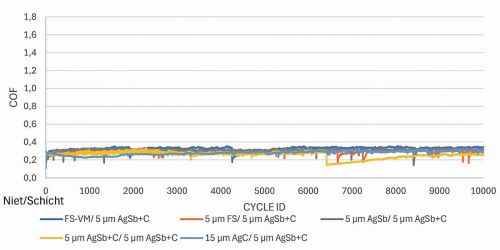 Fig. 6: Friction coefficients of a sample with an AgSb+graphite layer against different friction partners. Test parameters: 2 N, Ag rivet
Fig. 6: Friction coefficients of a sample with an AgSb+graphite layer against different friction partners. Test parameters: 2 N, Ag rivet
Increasing or decreasing coefficients of friction are an indicator that either the rivet layer or the main layer and the intermediate layer of the sample have been rubbed through. A possible interpretation for the blue curve would be, for example, that the rivet layer was worn through after approx. 1400 cycles, which is why the friction ratio between the copper and the FS layer on the sheet increased to approx. 1.2. After 6000 cycles, both the FS layer and the pre-silver layer on the sheet were rubbed through and the friction ratio between copper and nickel reached friction values of approx. 0.8. The AgSb+C layers on the rivets were not examined in more detail (Fig. 7).
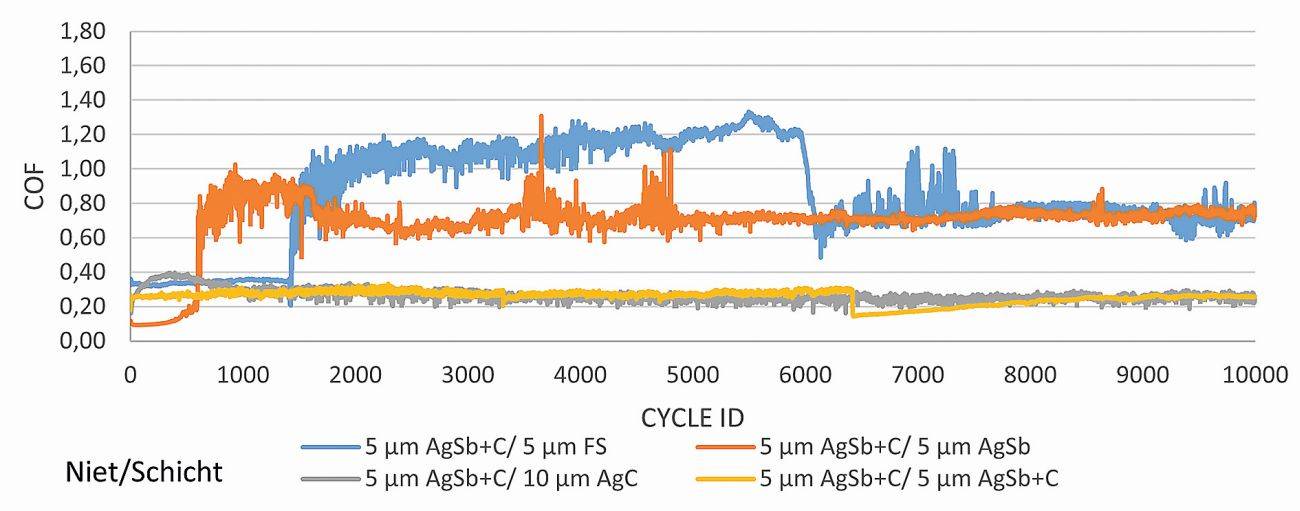 Fig. 7: Friction coefficients of samples with different Ag layers against friction partners with an AgSb+graphite layer. Test parameters: 2 N, Ag rivet
Fig. 7: Friction coefficients of samples with different Ag layers against friction partners with an AgSb+graphite layer. Test parameters: 2 N, Ag rivet
3D profiles of the tested coatings were created using a laser scanning microscope to determine the friction depths. The test results show that the fine silver and AgSb layers as well as their intermediate layers were completely rubbed through. The AgC sample was not found to be rubbed through, as the tested layer was 10 µm thick. The AgSb+C layer exhibited the lowest rubbing depth. Due to its hardness and good sliding properties, it exhibits very good wear resistance (Fig. 8).
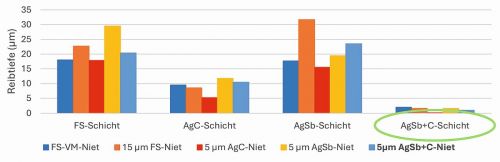 Fig. 8: Friction track depth of different tribological systems. Test parameters: 2 N, Ag rivet
Fig. 8: Friction track depth of different tribological systems. Test parameters: 2 N, Ag rivet
Conclusion
This study confirmed that the deposition of a coating with simultaneously increased hardness and good sliding properties can be achieved. The silver-antimony-graphite layers from the cyanide electrolyte were in the foreground and have shown improved wear resistance compared to fine silver, hard silver and silver graphite. At the same time, an increase in contact resistance was observed. This is negligible for the target application, as it only occurs at low contact forces (<5 mΩ from 60 cN).
The study also showed that the cyanide-free silver-antimony-graphite electrolyte and the cyanide silver-bismuth-graphite electrolyte offer additional possibilities for charging connectors for electric cars. Layers made from the cyanide-free silver-antimony-graphite electrolyte have the highest hardness of all the hard silver-graphite layers investigated, while layers made from the cyanide silver-bismuth-graphite electrolyte have the best contact transition resistances.
In further work, the influence of other working parameters on graphite incorporation, hardness, wear and electrical properties can be investigated. In addition, extended tribological investigations into the service life of the coatings - e.g. with higher contact forces and a greater number of friction cycles - can be carried out.
SOURCES
[1] Deutsche Gesellschaft für Galvano- und Oberflächentechnik e.V., Electroplated silver and silver alloy coatings including composites (2015).
[2 ]E. Vinaricky, K.-H. Schröder and J. Weiser, Electrical contacts, materials and applications. Fundamentals, technologies, test methods, 3rd edition, 262-265, Springer Vieweg Berlin, Heidelberg (2016).
[3] U.J. Möller and J. Nassa, Schmierstoffe im Betrieb, 2nd edition, 26, Springer-Verlag, Berlin, Heidelberg, New York, Barcelona, Hong Kong, London, Milan, Paris, Tokyo (2002).
[4] G. Khanra, S. Girikumar, D. Gangadhar, D. Mishra, T. Saravanan, S. Dineshraj and S. Sharma, Mater. Sci. Forum, 710, 326(2012).
[5] R. Arnet, A.-K. Egetenmeyer, H. Kappl and H. Willing, Galvanotechnik, 1, 21(2021).
[6] A.F. Karam, A. Franchini, D. Comte, A. Torrealba, S. Noël, A. Brézard-Oudot, C. Copper and J. Toran, 31st Int. Conf. Electr. Contacts-ICEC, 150 (2022).
[7] J. Korpiun and H.-J. Steeg, DE1240715B, 1967.
[8] I. Waldemar and R. Haemmerling, DE1496741A1, 1966.
[9] L. Stappers, C. Ntumba Ngoy , W. Zhang , M. Toben and J. Fransaer, J. Electrochem. Soc, 160, D137 (2013).
[10] K. Helle and F. Walsh, Trans. IMF, 75:2, 53 (1997).
[11] V. Behrens, T. Honig, A. Kraus, E. Mahle, R. Michal and K. Saeger, IEEE, 393 (1995).
[12] G. Behringer, H. Laub and S. Zjilstra DE2543082A1, 1979.
[13] H. Schreiner, Powder metallurgy of electrical contacts. Reine und angewandte Metallkunde in Einzeldarstellungen, vol. 20, 2, Springer, Berlin, Heidelberg (1964).
[14] P. Hahn-Weinheimer and A. Hirner and K. Weber-Diefenbach, Grundlagen und praktische Anwendung der Röntgenfluoreszenzanalyse (RFA), 3-5, Vieweg, Braunschweig, Wiesbaden (1984).
[15] U. Ritgen, Analytical Chemistry I, 273-279, Springer Spektrum, Berlin, Heidelberg (2019).
[16] L. Spieß, G. Teichert and M. Wilke, Die Materialcharakterisierungsverfahren Röntgenfluoreszenzanalyse (RFA) und Glimmentladungsspektroskopie (GDOES) im Alltag eines Werkstoffprüflabors, German Society of NDT - 2010 (DGZfP 2010), NDT.net Issue: 2011-01.
[17] J. Bauch and R. Rosenkranz, Physikalische Werkstoffdiagnostik, 2-3 & 84-85, Springer Vieweg, Berlin, Heidelberg (2017).
[18] S. W. Paddock, Mol. Biotechnol. , 16, 127(2000).
[19] K. Wetzig, Analytiker Taschenbuch 21, (ed.: H. Günzler et al.), 67, Springer Verlag, Berlin, Heidelberg (2000).
[20] E. Macherauch Praktikum in Werkstoffkunde, 9th edition, 49-51, Vieweg+Teubner Verlag, Wiesbaden (1990).