There are many ways to reduce energy consumption in electroplating, minimize theCO2 footprint and thus meet regulatory requirements and the expectations of major OEMs. Every step in the electroplating process offers opportunities for savings, some of which can be implemented with little effort and at reasonable cost.
Climate change is one of the greatest challenges of our time. Great efforts are being made to limit the rise in temperature to a maximum of 1.5 °C. In order to keep global warming to this 1.5 °C, globalCO2 emissions caused by humans should be brought to "net zero" in the foreseeable future. For example, the EU wants to be climate-neutral by 2050, and many companies, including well-known automotive OEMs, are even aiming for a much earlier target. The target described above has a significant impact on the metal industry. The entire supply chain from iron ore mining to the final product (e.g. fasteners, brake calipers, fluid lines, etc.) must first reduce and later eliminateCO2 emissions. Environmental, social and governance ("ESG") factors are an integral part of the business of a chemical technology company like Atotech. As a leading provider of sustainable coating systems, environmental protection has become a key driver of the company's strategy. Across all product lines, we strive to reduce the use of toxic substances and use less water, energy and raw materials, which also means less waste, fewer emissions and greater savings for our customers. The responsibility to promote ESG principles and best practices is taken very seriously. Therefore, customers are supported to the best of our ability in saving energy andreducing CO2. A typical customer process is batch galvanizing. Galvanizing is a very energy-intensive production process! The rectifiers consume a lot of electricity, and the heating and cooling of the various baths in pre-treatment, during the actual galvanizing process and in post-treatment cannot be ignored in terms of energy consumption. In all these areas, Atotech can make a major contribution to saving energy and thus reducingCO2 emissions with its modern coating processes and the use of special additional equipment. These possibilities are briefly examined in the following article, e.g. using various zinc-nickel coating technologies.Basis of the calculations
Atotech has developed a specialCO2 calculator to simulate the energy consumption in kWh for each stage of the electroplating process. This means that pre-treatment, electrolytic coating, post-treatment, drying and hydrogen de-embrittlement are considered separately. The specific heat capacity of the material to be coated, the part geometry and the associated rack or barrel technology, material throughput per unit of time, current yield, rectifier (voltage and current density), bath heating/cooling, bath temperature and current density are all taken into account.
heating/cooling, bath sizes, bath and part temperatures, furnace energy consumption, etc. The process-relatedCO2 sources, required current densities (rectifiers) and heating/cooling can be optimized with regard to their energy requirements. On the other hand, permanently installed plant equipment such as transport trolleys, pumps, extraction systems, ovens, etc. cannot be easily changed and is therefore not included in theCO2 calculation (Fig. 1). This still leaves almost 60 % of the coating process in whichCO2 savings can be directly influenced. The kWh consumption resulting from the calculation can be converted intoCO2 emissions using the respective emission factor of each country.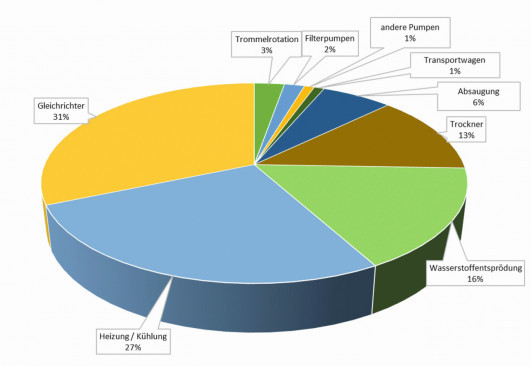 Fig. 1: Typical alkaline zinc-nickel drum coating
Fig. 1: Typical alkaline zinc-nickel drum coating
CO2 footprint of the entire value chain using the example of screw production
The theoretical consideration of the entire value chain from iron ore extraction to steel production, screw production and electroplating, including hydrogen de-embrittlement, shows a relatively small share of electroplating in totalCO2 emissions(approx. 5 %). Nevertheless, even this small proportion must be eliminated in the medium term in order to achieve the specified targets, meaning that the coating industry cannot avoid taking suitable measures to save energy. This results in the approach of considering each individual process step separately in order to generate optimized conditions everywhere.
CO2 savings in the pre-treatment process
Typical hot degreasing processes in electroplating require temperatures of up to 80 °C, which results in high energy requirements and therefore highCO2 emissions. It is easy to see that lower degreasing temperatures lead directly to energy savings and thus directly to a reduction in theCO2 footprint. For this reason, Atotech has developed low-temperature degreasers (UniPrep) that reach their optimum cleaning temperature in the 35-60 °C range. Depending on the hot degreasing output or target temperature, bath size and throughput, an energy/CO2 saving of 10-70% can be achieved in the degreasing process by switching to low-temperature degreasing. A nice side effect of UniPrep degreasers: They are extremely long-lasting, which leads to additional chemical and waste water reductions.
Savings in the zinc-nickel process
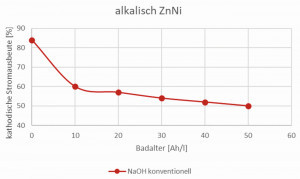
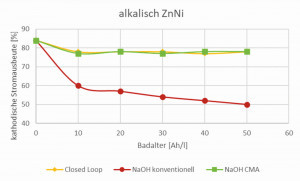
The calculations are based on a conventional alkaline zinc-nickel drum system for bulk material, in which the anodes are in direct contact with the electrolyte. In such a system, degradation products, e.g. cyanides, carbonates and various organic substances, are generated during the coating process through anodic oxidation, which has a negative impact on the overall process. The carbonates and organic degradation products increase the density and viscosity of the electrolyte. This leads to a drastic reduction in the cathodic current yield, accompanied by a reduced deposition rate (Fig. 2). The only remedy here is bath dilution (costs, waste water!) and carbonate freezing. In addition, cyanides form an extremely stable nickel complex, which results in increased nickel consumption, complicates wastewater treatment and leads to increased costs. The energy consumption andCO2 balance of such a typical alkaline zinc-nickel coating was defined as the basis for all subsequent zinc-nickel saving potentials.
Smart anode concept
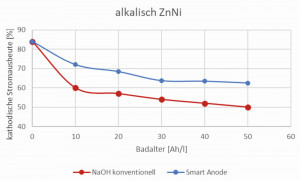
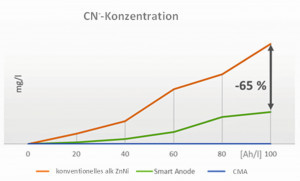
Smart anodes represent an initial concept. Special iron anodes are used here, which have a significantly reduced anode surface area compared to conventional anodes and therefore a higher anodic current density (> 10 A/dm2). This leads to reduced electrolyte degradation accompanied by less carbonate, reduced organic degradation products and less cyanide formation. The viscosity of the electrolyte is stable, the cathodic current yield remains at a higher level and the cyanide is significantly reduced (Fig. 3 and 4). As a result, the use of smart anodes results in lower chemical consumption, simpler wastewater treatment and, due to the improved cathodic current yield, an increased deposition rate, which increases overall productivity - all positive cost factors! The energy saving achievable based on the increased current efficiency has been calculated at 10-15%, equivalent to 10-15%CO2 reduction.
Membrane anodes
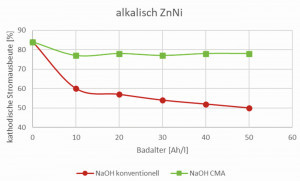
The patented CMA technology (Compact Membrane Anode) for alkaline zinc-nickel electrolytes prevents direct contact between the electrolyte and the anodes. This suppresses the anodic oxidation of the electrolyte components and prevents the formation of degradation products. This means that undesirable substances such as carbonate, cyanide and organic decomposition products are minimized or completely eliminated (Fig. 2 to 4). The membrane technology ensures that the electrolyte always approaches the state of a new deposit so that it retains its high cathodic current yield. This leads to an increased deposition rate, improved productivity and more consistent quality. CMA technology reduces resource consumption and wastewater treatment costs by ensuring cyanide-free operation. The high cathodic current yield also minimizes the energy required for coating and thus has a positive impact on theCO2 balance with savings of up to 45% (Fig. 5).
Closed loop
As explained above, the use of a membrane anode and a separate anolyte significantly reduces the formation of organic degradation products and cyanides are no longer formed. Carbonate formation is also significantly reduced. After a short time, all this leads to a stable, constantly high current yield, which only drops slightly compared to the initial value. The closed system (Closed Loop) also uses membrane technology and therefore behaves similarly to this in terms of current yield (Fig. 6). The CMA Closed Loop technology is a closed loop system that provides a comprehensive solution covering all aspects of the alkaline zinc-nickel plating process, including rinsing. It features the Compact Membrane Anodes (CMA) described above, customized chemical additives, a vacuum evaporator and a freezing unit, which together result in a significantly lower environmental impact, improved product quality, longer electrolyte life and ultimately reducedCO2 emissions. This innovative technology prevents the formation of organic degradation products such as cyanide and thus leads to a significant reduction in sludge formation. The integrated freezing unit plays a crucial role in purifying the electrolyte by removing accumulated carbonate. This ensures consistently high quality and improved performance throughout the coating process. In addition, the evaporation system separates the zinc-nickel electrolyte (additives and metals) from the water, whereupon these products can be returned to the electrolyte and rinses respectively. By returning the metals, chemicals and water to the plating circuit, the closed-loop CMA system drastically reduces the disposal and treatment of zinc-nickel wastewater by an impressive 90%, while significantly reducing NaOH, chemical and metal consumption. Rather than relying on downstream expensive and unsustainable wastewater treatment methods, the CMA Closed Loop system eliminates wastewater generation from the outset, setting new standards for the environment. The system enables higher cathodic current yields of 20 to 30 % compared to production with conventional anodes, so that the drum coating achieves up to 80 % cathodic current yield. Energy consumption can theoretically be reduced by up to 45% with CMA technology, which significantly improves theCO2 balance; 32% savings have already been demonstrated in practice. In addition, this technology results in significant savings by reducing NaOH, chemical and metal consumption. Due to its impressive results, the Atotech CMA Closed Loop technology was recently certified by TÜV Rheinland. This certification, which was achieved as part of the evaluation of electroplating plants at Atotech customers, quantifies the reduction of chemicals, rinsing and waste water as well as theCO2 footprint.
Acid zinc-nickel electrolytes
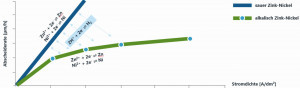
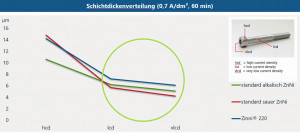
In general, the current yield of alkaline zinc-nickel electrolytes depends heavily on the cathodic current density. As the current density increases, the current yield decreases significantly. In an acidic electrolyte, on the other hand, the cathodic current yield is more or less independent of the current density, i.e. almost linear(Fig. 7). The increases in density and viscosity typical of alkaline electrolytes also do not occur in acid electrolytes, which means that the cathodic current yield remains at a constantly high level. A negative aspect of acid electrolytes in the past was insufficient metal distribution. This was primarily reflected in insufficient layer thicknesses in the low current density range and sometimes excessive layer thicknesses in the high current density range. With the introduction of the latest generation of acidic zinc-nickel electrolytes, e.g. Zinni 220, this deficiency has been remedied. Deposition in the low current density range is sometimes even better with Zinni 220 than with alkaline processes (Fig. 8). Today, it makes perfect sense to replace alkaline zinc-nickel baths with acidic ones in order to take advantage of the higher current yield while maintaining the same quality. This can significantly increase energy efficiency, with energy savings of up to 60 % depending on bath age, bath size, throughput, etc.
CO2 saving options in the post-treatment process
Thick-film, thin-film and black passivations are used at a wide range of temperatures. For example, some thick-film passivations have to be heated to up to 60 °C, while black passivations are sometimes cooled to below 20 °C. This enormous heating and cooling effort can be considerably reduced by processing passivations close to room temperature. Atotech has already successfully completed special developments for room-temperature passivations, so that the heating/cooling capacity could be reduced to zero. In figures: 100 % energy saving - 100 %CO2 reduction in the passivation process.
Conclusion
Reducing theCO2 footprint in electroplating is unavoidable. Major efforts must be made to achieve this in the short term. As mentioned above, there are already numerous methods available today to significantly reduce the energy requirements of each process step and thus make a contribution toCO2 reduction. However, in all the current discussions aboutCO2, other environmentally relevant issues such as compliance with regulatory requirements (e.g. REACh, etc.), saving chemicals and fresh water and reducing waste water should not be forgotten. And all this while maintaining quality and acceptable costs. Atotech also offers suitable solutions for this!