An energy-reduced anodizing process for the high-strength aluminium alloy EN AW 7075 has been developed as part of an AIF ZIM research project between the cooperation partners Forschungsinstitut Edelmetalle und Metallchemie Schwäbisch Gmünd (fem) and Diener & Rapp GmbH & Co.KG from Villingen-Schwenningen. The aim of the project was to develop a new type of anodizing process that can still guarantee the necessary coating properties with reduced electrical energy input. In total, energy savings of up to 25 % could be realized in the project.
Facts and problems High demand for electrical energy for anodization and bath cooling
A special feature of hard anodizing is the fact that temperatures around freezing point are required to maintain the tribological layer properties in the anodizing bath. This requires both high electrical power for anodizing and for cooling the anodizing baths. Hard anodizing of aluminium components generally requires bath voltages of around 30 to 60 volts, depending on the required coating thickness, with current densities of between two and four amperes per square decimetre. The electrical power required for anodizing is calculated from the product of the bath voltage and the anodizing current per anodizing time [1,2,3,4].
At the same time, around 30 % of the energy input during anodizing is released in the form of Joule process heat, which constantly heats up the anodizing bath and requires continuous cooling of the anodizing baths. Without cooling, the technical coating properties such as hardness and wear cannot be achieved. In refrigeration and cooling technology, the rule of thumb is that the electrical cooling energy required is around 20 % of the anodizing energy applied. In view of the current high electricity prices, both factors together represent an enormous cost factor for companies.
Solution to the problem Increasing the bath temperature and adding bath additives
By increasing the bath temperature and the resulting improved conductivity in the electrolyte, the bath voltage during anodizing can already be reduced by around 5 V at a temperature gradient of 10 °C. However, higher temperatures result in reduced abrasion. However, higher temperatures result in reduced abrasion and hardness properties, and corrosion resistance is also reduced. It was therefore necessary to investigate additional bath additives which, on the one hand, guarantee the tribological properties even at higher temperatures and, on the other hand, further minimize the electrical energy input [5,6,7,8,9].
Procedure
The primary objective was to investigate the influence of various electrolyte modifications with regard to the resulting tribological layer properties. Hardness measurements were carried out using the Fischer HM 500 device in accordance with ISO 14577. The abrasion resistance was determined using the NUS-ISO3CE testing device from Thermotec in Weilburg in accordance with ISO 10074. Topographical and morphological examinations of the anodized layers were carried out using scanning electron microscopy (SEM/EDX). The energy efficiency was calculated from measurable parameters such as voltage, current density, layer thickness and exposure time. The tests were carried out at electrolyte temperatures of 0/5/10 °C on sample sections (30 x 70 x 2 mm) of the alloy EN AW-7075 on a three-liter laboratory scale, initially on the fem.
The samples were pretreated using isopropanol, a sulphuric acid electrolyte with 185g/L sulphuric acid (ref. GS) was used as the base, and V2A stainless steel was used as the cathode material. The bath concentrations used for additive A (nitric acid) were 10/20/30 ml/l (ref. H1, H2, H3) and 8.5/17/26g/L for additive B (oxalic acid) in the form of oxalic acid dihydrate (ref. O1, O2, O3). The current density was 3A/dm2. The measured layer thicknesses were approximately 30-40 μm for an exposure time of forty minutes.
Measures and results Increasing the bath temperature and mode of action of bath additives
Figure 1 shows the reduction in electrical power after forty minutes of exposure time when the bath temperature is increased from 0 ° to 10 °C. This takes into account the effort required for anodization and the electrical effort required for cooling the anodizing baths, which in practice requires around one fifth of the electrical effort required for anodization. The electrical energy required can therefore be reduced by 20 % by increasing the temperature in the anodizing bath by 10 °C.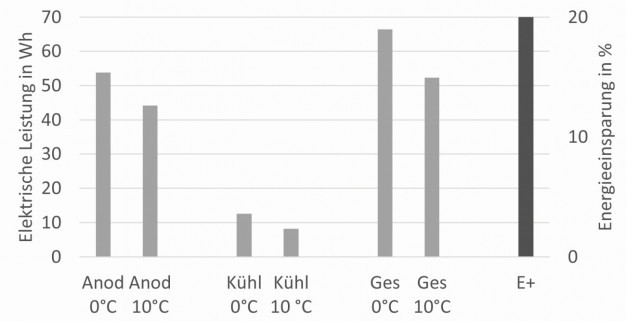 Fig. 1: Reduction in electrical energy required by increasing the temperature in the anodizing bath
Fig. 1: Reduction in electrical energy required by increasing the temperature in the anodizing bath
Figure 2 shows the reduction in bath voltage of around 5 V when the bath temperature is increased by 10 °C. Depending on the concentration, the bath voltage can be reduced by a further 4 V by adding nitric acid. Compared to a reference electrolyte operated at 0 °C, the sum of both measures can reduce the bath voltage by a considerable 7 V. It is known in the industry that oxalic acid in high concentrations in a sulphuric acid anodizing bath increases the electrolyte voltage, but in this concentration range, a voltage increase of only around 1 V was determined at a 10 °C temperature increase. Compared to a reference electrolyte that is operated at a working temperature of 0 °C, the bath voltage can be reduced by around 6 V by using both additives. Fig. 2: Reduction of the bath voltage by increasing the temperature and adding bath additives
Fig. 2: Reduction of the bath voltage by increasing the temperature and adding bath additives
Figure 3 shows the potential electrical savings of around 20 % by increasing the bath temperature by 10 °C alone. As can be seen, the savings potential could theoretically be increased to well over 30 % by adding nitric acid. As this significantly worsens the tribological properties, this had to be counteracted by adding oxalic acid. By using both bath additives, the total electrical energy savings can still be reduced by up to 25 %. Fig. 3: Energy savings by increasing the temperature and adding bath additives
Fig. 3: Energy savings by increasing the temperature and adding bath additives
Influence of bath additives on the hardening properties
Figure 4 shows that the hardening properties deteriorate as expected when the temperature is increased. The extraordinarily high standard deviations should be noted in this context. These are due to the high surface roughness in some cases, as well as the existing morphological layer properties (porosity, microstructure). Additions of nitric acid at a bath temperature of 10 °C show no significant reduction in hardness properties depending on the concentration. Using oxalic acid as a bath additive, on the other hand, a significant increase in hardness can be measured. Both additives taken together lead to Vickers hardnesses that are comparable to those of a standard hard anodizing.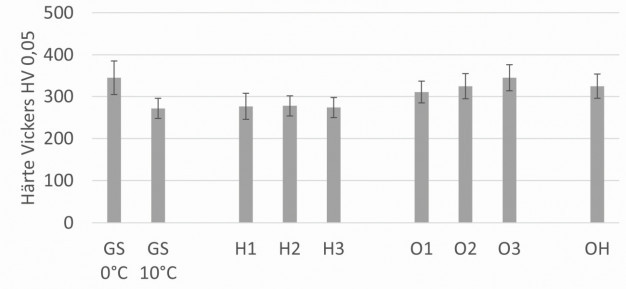 Fig. 4: Hardness measurements according to Vickers
Fig. 4: Hardness measurements according to Vickers
Influence of bath additives on abrasion resistance
Figure 5 shows that the abrasion resistance and the expected wear resistance deteriorate slightly with the addition of nitric acid. However, depending on their concentrations, both additives taken together lead to wear values that are quite comparable to those of a standard hard anodizing. Fig. 5: Abrasion resistance according to ISO 10074
Fig. 5: Abrasion resistance according to ISO 10074
The NUS-ISO3CE wear and abrasion tester from Thermotec (Fig. 6) can be used to determine the abrasion behavior of anodic oxide films, coatings, plastics and other products. A slip ring rotates by 0.9° with each back and forth movement, allowing the sample to be constantly reloaded on an unloaded surface area, which is not possible with conventional methods. The slip ring also loads the sample from below, whereby the abrasion dust immediately falls downwards, i.e. does not remain on the sample and therefore has no detrimental effect. This ensures significantly better reproducibility compared to the conventional Taber abrasion test method. Fig. 6: Abrasion test device NUS-ISO3CE from Termotec
Fig. 6: Abrasion test device NUS-ISO3CE from Termotec
A complete 360° rotation of the grinding wheel requires a total of 400 friction wheel movements; 40 double strokes per minute are performed as standard. All types of different abrasive papers can also be used. The samples to be examined should be flat and have a minimum size of approximately 30 x 50 mm. The sample thickness can vary between 0.8 and 4 mm.
Overview of hardness properties and energy savings
Figure 7 summarizes the influence of the bath additives on the hardening properties in comparison with the expected energy saving potential. By using nitric acid alone and increasing the temperature, an energy reduction of over 35 % would be possible, but the hardness values would be significantly reduced. By adding oxalic acid or both additives together, Vickers hardnesses of over HV 300 can be achieved. Savings potentials of 20 % or more are quite realistic. Fig. 7: Energy savings in comparison with the hardness properties
Fig. 7: Energy savings in comparison with the hardness properties
Discussion Influence of nitric acid
The mode of action of nitric acid as a bath additive cannot yet be fully explained; presumably zinc, copper and iron components are preferably dissolved directly from the matrix during anodization, thereby suppressing the formation of molecular oxygen. This would make more electricity available for the formation of an aluminum oxide layer. This would increase the efficiency, but this can only be proven by precisely determining the weight of the oxide layer and by measuring the layer thickness in the cross-section. If, as a result, less oxygen were developed at the anode, the formation of cavities in the oxide matrix should also be reduced, which in turn would favor the hardening properties. Another explanation for the reduced electrical output due to nitric acid could be related to an increase in the conductivity of the electrolyte, which is currently being investigated. Using nitric acid as a bath additive in everyday practice also requires continuous analytical bath monitoring with regard to the complex acid mixtures present.
Influence of oxalic acid
The positive influence of oxalic acid in anodic oxidation has already been investigated many times in the past. Presumably, anions are adsorbed on the pore walls of the anodized layer and therefore reduce the chemical re-dissolution of the oxide layer. In this way, harder and abrasion-resistant coatings are obtained even at higher bath temperatures. By increasing the temperature from 0 to 10 °C and adding oxalic acid to a base electrolyte, potential savings of 20 % are realistic. Vickers hardnesses of 300 or even much higher can be achieved. The abrasion resistance achieved reaches the values of conventional hard anodized coatings.
Conclusion
Intensive research has led to significant technical and scientific progress in classic hard anodizing processes in the anodizing industry. The consumption of electrical energy can be reduced by around 20 to 30 % simply by increasing the temperature in the anodizing bath by 10 °C and adding nitric acid and oxalic acid. The coating qualities are on a par with those of standard hard anodizing.
The ZIM KK511910MP1 project was funded by the Federal Ministry for Economic Affairs and Energy via the AIF of the Central Innovation Program for SMEs (ZIM).
Literature
[1] Hübner, W.; Speiser, C.: Die Praxis der anodischen Oxidation des Aluminiums, 4th edition, Aluminium-Verlag, Düsseldorf, (1988)
[2] Pinner / Sheasby: The Surface Treatment and Finishing of Aluminum and its Alloys, 5th edition, ASM International, USA, Finishing Publications Ltd, Middlesex, England, (1987)
[3] Brace, A. W.: Hard Anodizing of Aluminium, Technicopy Ltd, Stonehouse, England, (1987)
[4] Jelinek, T. W.: Surface treatment of aluminium, Eugen G. Leuze Verlag, Saulgau, (1997)
[5] Najafi, M.: Characterization of the influence of organic compounds during anodizing of the aluminum alloy EN AW 7075, Master thesis, TU Ilmenau, (2021)
[6] Morgenstern, R.; Sieber, M.; Scharf, I. and Lampke, T.: Influence of foreign acids on the anodizing of copper-containing aluminum alloys, Wotech, (2018)
[7] Hübner, W.W.G.: The anodic oxidation of aluminum in differently composed oxalic acid solutions, Diss., ETH Zurich, (1948)
[8] Hashimoto, T.; Zhou, X.; Skeldon, P. and Thompson, G. E.: Structure of the Copper-Enriched Layer Introduced by Anodic Oxidation of Copper-Containing Al Alloy, Electrochimica Acta, vol. 179, (2015)
[9] Mock, C.; Kölle, S.; Schmid, K.: Fraunhofer-IPA, Stuttgart, Womag 11, (2015)


