If hydrogen is passed over a catalytic converter, a considerable amount of heat energy is released during combustion. The only waste product is pure water. The idea of capturing and thermally utilizing hydrogen that is produced in industrial processes and escapes unused into the ambient air is an obvious one.
When anodizing aluminium, hydrogen is formed at two key process points. Firstly, through aluminium dissolution during pickling, and then at the cathodes in the anodizing bath during anodizing. The latter was investigated in more detail in a research project.
Technical background
The utilization of hydrogen is based on extracting it from the cathodes with the aid of a textile coating and mixing it with air to form a gas mixture. For safe operation, an H2 concentration of < 4.4% by volume (LEL) below the explosion limit must be maintained.
During the technical implementation in a certified anodizing plant, an external temperature of 220 °C was measured on a tube bundle catalytic converter, allowing a compression bath to be additionally heated at 96 °C. Another positive side effect of the process is the reduction of sulphuric acid aerosols in the ambient air. Continuous measurement of the hydrogen concentration and control of the extraction air are crucial for safe handling.
Further information on the project can be obtained directly from fem in Schwäbisch Gmünd.
R&D partner
- Riedel & Soelch GmbH
- Nuremberg metal finishing plant
- fem | Research Institute Precious Metals + Metal Chemistry
- Neoxid GmbH Neuss
- Alcon Aluminum Consult GmbH Düsseldorf
Contact at
fem (Research Institute Precious Metals + Metal Chemistry),
Katharinenstraße 17, 73525 Schwäbisch Gmünd, Stefan Funk, Tel.:07171-1006-503,
Acknowledgements
The project ZIM ZF 4215103Z G6 of the Forschungsvereinigung Verein für das Forschungsinstitut für Edelmetalle und Metallchemie (fem) was funded by the Federal Ministry for Economic Affairs and Energy via the AiF of the Central Innovation Program for SMEs (ZIM) on the basis of a resolution of the German Bundestag.
Copper for electromobility
Combined with an efficient energy grid, electrification, automation and digitalization can provide new approaches to meet the future challenges of mobility. Copper is one of the key materials enabling this transition.
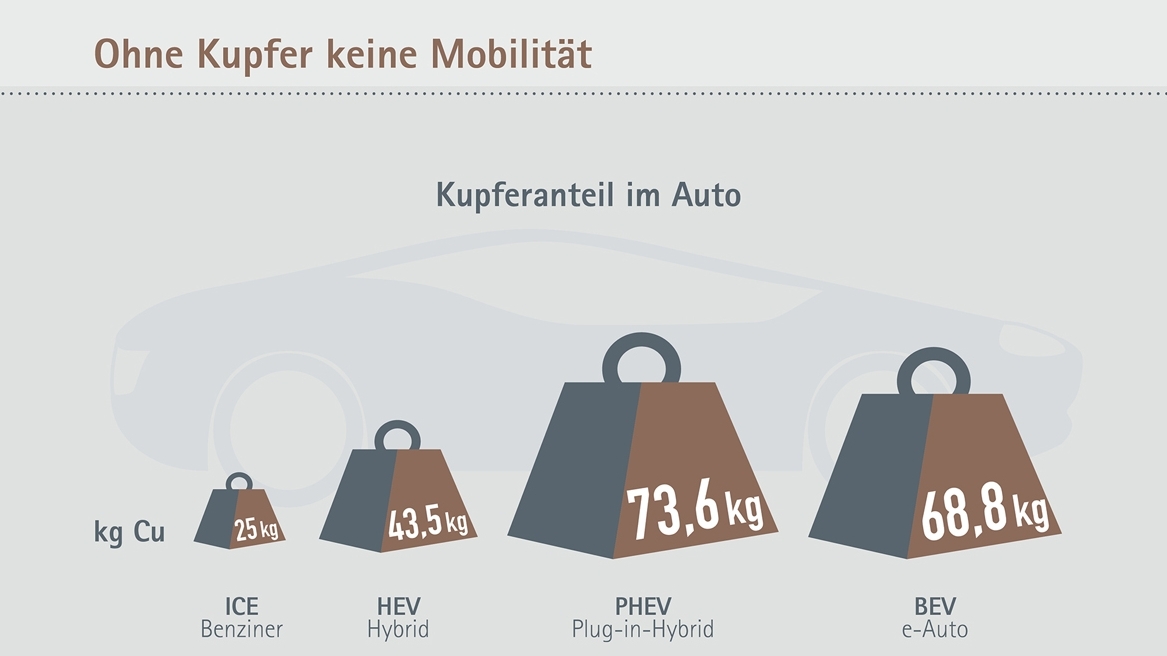
On average, an electric vehicle contains almost three times as much copper as a vehicle with a combustion engine. Half of this copper is found in the battery. The generation of electricity from renewable energies and the infrastructure required for charging electric vehicles also creates a high demand for copper.
The lithium-ion battery alone consists of around 18 percent copper, as the cathode is always made of aluminum and the anode of copper as a carrier material. At least one drive motor and one inverter contribute to the fact that there is easily three times as much copper in such a vehicle as in a conventional vehicle with a combustion engine - namely around 25 kg in the average mid-range petrol vehicle. In the future, it is even expected to be up to 40 kg, as the desire for more comfort means that many small electric motors containing copper will be needed. The greatest increase in copper weight is to be expected in the area of the new components added to electrified vehicles - drive energy storage, electric motor, high-voltage electrical system, power electronics, etc. In the case of a plug-in hybrid, this can be more than 70 kg of copper in the mid-range, while the e-car is just under that.