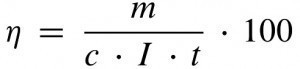Question: Our nickel electrolyte has a volume of 8000 liters. In order to maintain the highest possible, consistent quality, we would like to clean the bath continuously with activated carbon. We have had some loud discussions about this. Some of the electroplaters swear by filter cartridges, while others reject them outright and demand continuous selective cleaning coupled with discontinuous activated carbon cleaning, which should be carried out once or twice a year. So who is right?
As is so often the case in electroplating technology, there is no one-size-fits-all answer, as there are many parameters and points of view that count. Or, as they say in practice: "In electroplating, 1+1 is not always 2. Sometimes it's 1.5, sometimes 2.5, and if you're lucky, it's even 2."
The task of activated carbon cleaning
Activated carbon purification is normally carried out to remove unwanted decomposition products from the electrolyte. These predominantly organic compounds accumulate on the large surface of the activated carbon and can therefore be physically removed from the electrolyte. The problem is - to put it bluntly - that neither the activated carbon nor the substances themselves know what needs to be removed from the bath and what does not. This means that everything in the bath that accumulates enough on the activated carbon is removed, including important ingredients. As a result, your consumption of organic additives will increase significantly with continuous filtration. Activated carbon is used both for existing problems and - as you intend - as a preventative measure. The problems that can be solved with activated carbon treatment are as follows: Low deposition rate; Lack of throwing power; Double nickel; Brittle coatings and Lack of adhesion (bubbles, flaking). The first three points are mainly due to an overdosage of organic additives. The last two points can be caused by degradation products. The literature specifies pH values of 3.0 to 3.8 at which treatment should be carried out [1]. In discontinuous treatment, activated carbon is added directly to the electrolyte. The literature data also varies here and ranges from a concentration of 6 to 10 g/L [2]. The specified reaction time is between 30 minutes and two hours with continuous stirring. The effort involved is therefore considerable, especially when you consider the work required before and after.
Backup electrolyte
Some electroplating shops work with two electrolytes. One is in use, the second is in maintenance or standby mode. The advantage of this is that you can react very quickly to problems in production. Depending on the bath volume, the active electrolyte can be replaced quickly and you have a perfectly adjusted nickel electrolyte in use again. You then have time to clean and adjust the formerly active bath. This practice is also used in some companies as a preventive measure every six to twelve months without any problems. The disadvantages of this practice are obvious. The higher the bath volume, the higher the running costs. Not only do you have a second electrolyte that is of no use to you during the waiting period, you also have heating and maintenance costs. This is offset by unknown costs in the event of complaints and downtime. Electroplating companies usually only opt for such a solution when these costs are already on the table.
Cleaning with filter cartridges
Normal filter cartridges and similar methods are common practice. Until 10-15 years ago, it was relatively normal to use an activated carbon cartridge. Back then, it was easier to afford the higher organic consumption than it is today. The structure looks like Figure 1. In order to reduce costs, the first measure was to reduce the feed to the activated carbon cartridge. The second step was to use a separate pump that was only switched on for a few hours during work breaks, for example at night or at weekends. However, the success of the cleaning process is reduced to the same extent as the costs.
Selective cleaning in the bypass
Selective cleaning is the separation of metallic impurities from nickel electrolytes at low current densities (0.1 to 0.3 A/dm2). This allows not only foreign metals to be removed, but also organic impurities. Excess brighteners can also be removed in the same way. Many nickel electrolytes, which are cleaned continuously and selectively, make the troublesome and costly activated carbon treatment superfluous. It is advisable to use only flat corrugated, but preferably completely flat sheets, which are either corrugated or slightly beveled for stability. These have the advantage that, if different impurities are present, these impurities can be removed in selected, i.e. selective, voltage or current ranges, whereby the entire surface is then available for removal [2]. While tramp metals are usually removed at current densities of around 0.1 to 0.3 A/dm2, particularly low current densities (e.g. 0.01 to 0.03 A/dm2) are usually required for the removal of organic impurities.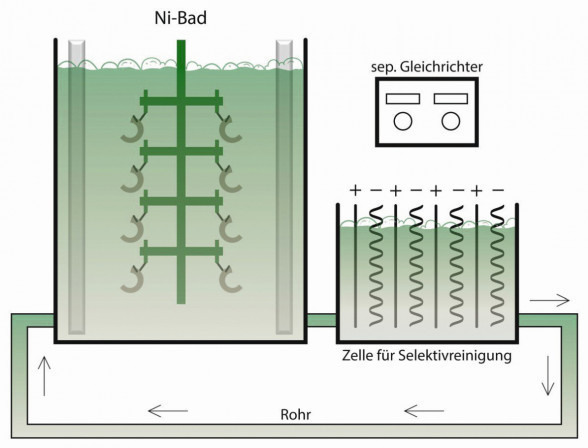 Fig. 2: Selective cleaning in the bypass
Fig. 2: Selective cleaning in the bypass
Selective cleaning can also be carried out in the bypass (Fig. 2). In this case, the electrolyte is pumped into a separate electrolysis cell during operation. Here, cathode plates and anodes are arranged close together and connected to a separate rectifier. This selective cleaning is particularly advantageous for high-performance electrolytes, for example in strip electroplating, to stabilize the process. Here too, it is important to keep an eye on the costs. Continuous selective cleaning may be appropriate for high-performance electrolytes, such as nickel sulphamate electrolytes in strip plating lines. With classic nickel sulphate electrolytes, however, this can also be done discontinuously during work breaks or made dependent on analyses and Hull cell plates [3]. With a Hull cell, many properties of a nickel layer and thus the electrolyte state can be tested in a standardized manner with little effort. This means that you always have the same conditions and the cyclically performed tests are comparable.
The following properties can be tested:
- Layer thickness in different current density ranges
- Coating thickness distribution
- General optical condition (e.g. streaks, cloud formation)
- Gloss level
- Gloss range
- Metallic, but also organic impurities
- Ductility
- Hardness
- Efficiency / current yield.
The efficiency is best determined by the metal mass. For this purpose, the Hull cell sheet is weighed before and after coating and the difference is used in Faraday's formula.
As most nickel electrolytes are heated, the Hull cell test should also be carried out in a heated cell. For high-performance baths, nickel plating is carried out for 10 minutes at 2 A/dm2, for standard baths for 15 minutes at 1 A/dm2. Nickel plating is carried out on (usually brushed) brass cathodes. The use of brass is due to the visually clear distinction from the nickel layer. Pre-treatment is generally carried out in the same way as in production. It is important that the sheet is completely wetted with water before coating and that this H2Ofilm is retained without wetting. After coating, the sheet metal is rinsed well and dried. Deionized water is used to avoid water stains. The Hull cell sheets are usually photographed for documentation purposes. A good practice to detect ductility problems (which can be caused by excessive chloride content and organic impurities) is to bend the lower corner of the high current density area by 90° with pliers. A cracking sound can be a corresponding indication.
 Fig 3: A simple Hull cell made of Plexiglas
Fig 3: A simple Hull cell made of Plexiglas
Reprocessing of electrolytes
Even with perfect selective cleaning, problems can occur, for example due to carryover, overdosing and other contamination, e.g. due to the product to be coated itself. For this reason, a well-equipped electroplating shop should also have facilities for treating an electrolyte. The end of the service life is reached as soon as the electrolyte no longer works properly and the problems cannot be rectified in the production tank. Then the only option is to dispose of it or reprocess it.
The more electrolyte volumes there are, the more worthwhile reprocessing tends to be, as there is hardly any difference in set-up and working time between, for example, 1000 liters and 8000 liters of electrolyte. The procedure depends on the exact type of contamination. As a guide, you can proceed as follows:
- If Me contamination is present: If it needs to be precipitated or reduced, this should be started.
- Then filter and, if necessary, carry out selective cleaning. Some of the organic matter is already broken down during this process.
- Activated carbon cleaning is carried out to remove the organics.
- As not all organic matter remains on activated carbon, UV treatment should also be carried out.
Treatment with UV light can also help with microorganism infestation. However, success should be measured by adding a little acetic or citric acid to a negative sample. In this case, the supposedly killed microorganisms can come back to life. If the organic matter is to be destroyed by means of UV oxidation, the TOC value (total organic carbon) is checked. The treatment takes place in several stages, starting with pre-oxidation, followed by main oxidation, combination processes and final treatment. In individual cases, it can be checked whether metal impurities can be removed with selective exchangers (a form of ion exchanger). In this case, resins are used that have a particularly high affinity for the desired metal and therefore do not bind the nickel.
Conclusion
To our knowledge, there is no perfect solution that works flawlessly in all conceivable cases. In your particular case, we would favor selective cleaning and initially operate it discontinuously, whereby regular monitoring of the coating quality is necessary. In order to be prepared for an emergency, the optional operation of a filter cartridge with activated carbon would also be conceivable. In our opinion, however, it seems much more important to be equipped for reprocessing the electrolyte.
Literature
[1] Die galvanische Vernicklung, Eugen G. Leuze Verlag GmbH & Co. KG, 1st edition 1984, ISBN 3-87480-009-1
[2] Online course "Galvanic nickel plating"; https://www.galvanotechnik-for-you.de/uebersicht-kurse/die-galvanische-vernickelung/
[3] Hull cell, Eugen G. Leuze Verlag GmbH & Co. KG, 1st edition 2007, ISBN 3-87480-224-8