Question: We have a rack system in which only steel parts are nickel-plated to a mirror finish. The nickel electrolyte consists of two tanks with three stations each. The volume per tank is 6000 liters. Our problem is that the plant is running at 100% capacity and we sometimes have problems coating customer orders on time. The bottleneck is nickel plating. Pre-treatment could cope with double the capacity, but nickel plating (on average around 12-20 µm) takes time. Unfortunately, it is not possible to extend or even build a new plant.
 Nickel bath with movement We would therefore like to speed up the nickel plating process. We are currently using a Watts electrolyte. It is operated at 60 °C, a pH value of 4.2 and a nickel content of 65 g/L. Due to the geometry of the parts, we run the electrolyte at 1 to 1.5 A/dm2. Depending on the required coating thickness, a product carrier remains in the station for 40 to 100 minutes. Our aim is to halve this time. We are just not sure whether it is better to optimize the current electrolyte or to use a different type.
Nickel bath with movement We would therefore like to speed up the nickel plating process. We are currently using a Watts electrolyte. It is operated at 60 °C, a pH value of 4.2 and a nickel content of 65 g/L. Due to the geometry of the parts, we run the electrolyte at 1 to 1.5 A/dm2. Depending on the required coating thickness, a product carrier remains in the station for 40 to 100 minutes. Our aim is to halve this time. We are just not sure whether it is better to optimize the current electrolyte or to use a different type.
Answer: We assume that you have already taken into account the changes to the periphery. This applies to the rectifier, power supply, anode and cathode bars as well as the fact that the anodes have to be refilled twice as often if the deposition rate is doubled.
As far as the anodes are concerned, the jump from 1.5 to 3.0 A/dm2 makes no appreciable difference to solubility or passivity. It is only important that the anode rods and baskets are generally designed for this. If you want to work with higher currents, the chloride content may have to be set higher, which in turn has a negative effect on the ductility of the layer. An often underestimated aspect of 100 % capacity utilization is the lack of maintenance work. Even if you schedule time for this, in the long term there is a risk that maintenance work will be sloppy due to an overload of employees. This can affect the addition of chemicals, maintenance of anodes, contacts, racks and other areas. Any resulting rework will further exacerbate your situation.
As you mention, there are two options here. An adapted Watts electrolyte or a high-performance electrolyte, whereby we recommend a nickel sulphamate electrolyte. Both aspects are explained below.
Modified Watts electrolyte
 Hull cellTheadvantages of modifying the electrolyte are obvious. You can continue to use the same chemistry, carry out the same analyses and the resulting chemical costs are kept within limits.
Hull cellTheadvantages of modifying the electrolyte are obvious. You can continue to use the same chemistry, carry out the same analyses and the resulting chemical costs are kept within limits.
In principle, there are two problems to solve:
- Avoid burns on tips and edges.
- Ensure uniform layer build-up (dog-bone effect).
Classic nickel electrolytes are already suitable for 3 A/dm2. Modified nickel sulphate electrolytes can even be operated at 5-7 A/dm2. Ion transport plays an important role here.
The following methods are used in conventional electroplating technology to get to grips with the problem of ion transport:
Increase the electrolyte temperature:
Modern bath additives can withstand permanent temperatures of 70 °C. Increasing the temperature improves ion mobility, conductivity and solubility in equal measure, although the ion mobility is weakened by higher additions.
Increase nickel concentration:
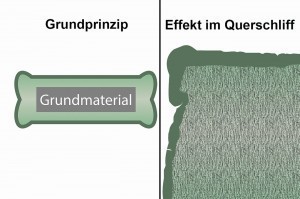 Dog bone effectThemetal content can be increased to 75 g/L. If necessary, you can also set 80 g/L. However, we would like to point out that this will of course also increase the carry-over and the rinsing criterion may no longer be achieved. This can be partially compensated for by recirculating more from the first rinse due to the higher electrolyte temperature.
Dog bone effectThemetal content can be increased to 75 g/L. If necessary, you can also set 80 g/L. However, we would like to point out that this will of course also increase the carry-over and the rinsing criterion may no longer be achieved. This can be partially compensated for by recirculating more from the first rinse due to the higher electrolyte temperature.
Convection:
In addition to optimizing temperature and chemistry, in many cases a lot can be done to optimize convection. The aspect of uniform layer structure can even be taken into account. This means that there is a stronger flow at the points where the layer thickness is lower. However, this cannot always be achieved perfectly. With appropriate additives, it should even be possible to achieve air movement. You should always clarify this with the chemical supplier beforehand. Although it is sometimes possible to improve the fabric movement, we assume that this has already been exhausted in the past.
Bath additives can possibly help to improve the layer thickness distribution. If this has already been exhausted, we recommend blanking off high-current areas.
Compositions
Nickel: 75 g/L
Chloride: 20 g/L
Boric acid: 50 g/L
pH value: 4.2
Temperature: 70 °C
Cathodic current density: 3 A/dm2
Efficiency: approx. 98 %
In order to set the additives optimally, you should run Hull cell sheets with the current setting and compare them with the modified parameters and make any necessary adjustments.
Nickel sulphamate electrolyte
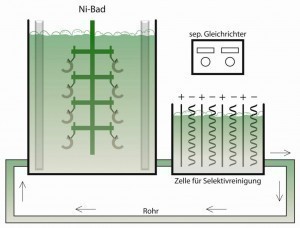 Nickel bath selective cleaningNickel sulphamate electrolytesare mainly used in electroforming, electroforming, strip nickel plating and heavy nickel plating. Applications include the production of nickel foils, casting and pressing molds (e.g. for CD-ROMs) and printing plates.
Nickel bath selective cleaningNickel sulphamate electrolytesare mainly used in electroforming, electroforming, strip nickel plating and heavy nickel plating. Applications include the production of nickel foils, casting and pressing molds (e.g. for CD-ROMs) and printing plates.
Nickel coatings made from nickel sulphamate electrolytes generally exhibit lower internal stresses than coatings made from an additive-free sulphate electrolyte. The type and level of internal stresses can be influenced by electrolyte additives and by the working conditions. Electrolyte additives include hardeners, stress reducers and wetting agents. These are largely the same organic substances that are also used in nickel sulphate electrolytes. Nickel sulphamate electrolytes allow the deposition of thick, low-stress nickel layers at high deposition rates. One disadvantage, apart from the high preparation costs and ageing due to the decomposition of sulphamate to ammonium sulphate, is the high sensitivity to impurities. They therefore require particularly pure chemicals and selective cleaning in the bypass.
Nickel sulphamate (Ni(SO3NH2)2 - 4H2O) is usually supplied as a liquid concentrate. The advantage of this metal salt is its very high solubility. This allows a nickel content of up to 165 g/L to be achieved. Very high current densities are therefore possible.
Compositions
350 g/L nickel sulphamate (Ni(SO3NH2)2 - 4H2O)
10 g/L nickel chloride (NiCl2 - 6H2O)
35 g/L boric acid (H3BO3)
Additives
a) 35 mg/L saccharin (C7H5NO3S)
b) 5 g/L naphthalene tri-sulfonic acid
Working conditions
Temperature: 40-60 °C
Current density: 5-20 A/dm2
pH value: 3.5-4.5
Anodes: pure nickel / electrolyte nickel
Analysis target values
Nickel: 60-75 g/L (65)
Chloride: 3-5 g/L (3)
Boric acid: 30-45 g/L (35)
With high-speed electrolytes, the boric acid content can be increased to up to 50 g/L and the nickel sulphamate content can be up to 600 g/L. These high-performance baths are operated at up to 70 °C and the electrolyte is circulated very strongly. This should not be necessary for your application. An electrolyte temperature of 60 °C is generally regarded as optimal for obtaining low-stress coatings. Temperatures above 70 °C should be avoided, as the sulphamate begins to decompose at these temperatures, producing ammonium chloride and ammonium sulphate.
The costs of the sulphamate electrolyte are considerably higher than those of a sulphate electrolyte. Mixed sulphamate-sulphate electrolytes are also used for electroforming and nickel-plating technical articles.
A particular challenge will be to achieve the same appearance with the sulphamate electrolyte that you currently guarantee your customers. Here we recommend extensive test series and consultation with the customer. This concerns not only the appearance, but also other coating properties such as hardness, ductility, roughness, corrosion resistance, etc.