Question: During internal checks, we discovered that there are cyanide compounds in the wastewater treatment of chromium(VI). Further investigations showed that the cyanide is carried over from the triple cascade rinses of the cyanide zinc bath into the chromating process. There is a stand rinse between the electrolyte and the cascade. The "stand sink" is currently just a drip tray with no water in it. Now we have the difficulty that, on the one hand, we are unsure how to avoid the problem and, on the other hand, how to properly detoxify the mixture; after all, cyanide must be oxidized and chromium(VI) reduced. One idea was to add the oxidizing agent directly to the cascade rinses in order to achieve direct or at least preliminary detoxification. As we also have a hardening plant, there is also nitrite in our waste water. Could the nitrite in the mixture also be detoxified by oxidation or reduction?
Answer: Proper wastewater management and flushing should prevent such problems from occurring. However, practice shows that this can certainly be the case. As we assume that you have a separate cyanide detoxification system, the first measure is to prevent carryover.
Retention of cyanides
First of all, it is clear that you have a flushing problem. Even if only cascade rinsing is used, the rinsing result should be sufficient under normal circumstances. This means that either the volume is too low, the rinsing times are insufficient or - most likely - the supply of fresh water is insufficient.
You should first check whether the times and/or the water dosage have been changed. If so, question the reasons and assess whether the disadvantages that have arisen outweigh the disadvantages.
The next measure would be to restore the original economy sink to its original function. If the return to the zinc bath is no longer sufficient due to the dilution and you generally want to reduce the cyanide content in the wastewater system, there is the option of pre-detoxification by anodic oxidation in the bypass. In this case, an electrolytic cell is connected to the economy sink, in which cyanide is oxidized anodically and zinc is deposited cathodically. Among other things, this saves fresh water and can significantly reduce the cyanide content of the rinse.
However, we strongly advise against adding oxidizing agents to the sinks. These would carry over into the chromating process. Depending on the drain and pipework, these oxidizing agents can get into the circulation water and damage the resins of the ion exchangers. It is also no longer possible to return them to the zinc bath. In addition, you may also oxidize the zinc layer and the chromate coating may no longer be applied, and traces of zinc corrosion may even form, which is the opposite of what you want to achieve with the coating (corrosion protection).
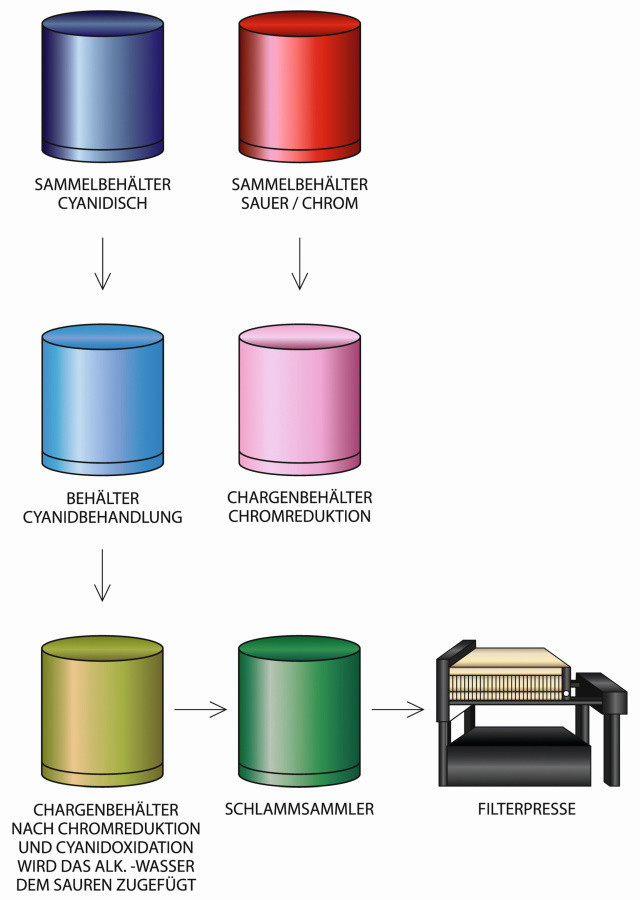 Normal process for cyanide and chromium-containing wastewater
Normal process for cyanide and chromium-containing wastewater
Nitrite in waste water
Even if the hardening shop is located on the same premises and the waste water is possibly treated in the same rooms as the electroplating shop, it should be separated from the rest, at least in a separate collection tank. Especially with RedOx reactions, it is often a bad idea to mix everything together and hope for the best, e.g. because you may not get a clear potential jump and - depending on the quantity ratio - not know what is happening. This approach is usually only beneficial for precipitation reactions after all previous reaction steps have been completed, e.g. destruction of complexes, removal of ammonium, etc.
There are two ways to destroy nitrite: You can oxidize it to nitrate or reduce it, after which you get carbon and nitrogen compounds. Both methods have advantages and disadvantages.
If you are working with hydrogen peroxide anyway, oxidation is a good option:
HNO2 + H2O2 → HNO3 + H2O
This produces nitric acid, which is later neutralized. The residence time should be at least 15 minutes. With the correct dosage, the formation of nitrous gases can be minimized or avoided altogether.
Nitrite can be reduced with dihydrogen sulphite (sulphurous acid, H2SO3), urea (CH4N2O) and aminosulphuric acid (amidosulphonic acid, H3SO3N):
2 HNO2 + 3 H2SO3 → N2+ 3 SO42- + 6 H+ + H2O
2 HNO2 + CH4N2O→ 2 N2 +CO2 + 3 H2O(reaction only takes place from 60°)
These processes are more expensive and - in the case of urea - involve a high energy input.
Detoxification of cyanide and chromium(VI)
In the case of separate wastewater, the cyanide-containing wastewater is oxidized and the chromium-containing water is reduced in another treatment tank. Once both processes have been completed and the excess oxidizing agents have been removed from the wastewater formerly containing cyanide, these can be mixed and further treated together with other water, for example from pickling and degreasing processes.
There are several processes for both reactions [1-3], which will not be discussed in detail here. If you look at these, you will see that hydrogen peroxide can be used to reduce chromium and oxidize cyanide. It can also be used to oxidize nitrite, as described. However, we consider this procedure to be very critical, as we will explain below.
In the presence of nitrite, nitric acid is formed, as explained. This means that the pH value drops. This is even necessary for chromium reduction with H2O2, as this only takes place at pH < 3.5. Depending on the ratio of chromium to cyanide and the original pH value, hydrocyanic acid can form here, provided the cyanide has not already been converted into hydrocyanic acid during chromating, i.e. production. This should be mentioned in case there are possibly several sources for the problem described and we do not know the pH value of the CN-Cr(VI) mixture.
In addition, the more wastewater you mix, the more unknowns you are working with. One potential problem is substances that have a catalytic effect on hydrogen peroxide. This applies to copper, to name just one popular example. Such substances can lead to spontaneous reactions of H2O2 and the waste water makes its way out of the treatment tank in an explosive manner.
We therefore recommend focusing on the oxidation of cyanide in the mixture (without nitrite). The chromium can then be reduced, as a reverse reaction from cyanate to cyanide is quite impossible.
As a comparatively small amount of cyanide is involved, we recommend an experiment with Karoat (2KHSO5 KHSO4 - K2SO4). The acidic triple salt is somewhat more expensive than the usual agents, but is easily soluble and has no disadvantages such as AOX formation, corrosion etc.
After cyanide oxidation, the chromium(VI) can be reduced as usual.
Literature
[1] Environmental technology part 1 - Wastewater treatment: https://www.galvanotechnik-for-you.de/uebersicht-kurse/umwelttechnik-teil-1-abwasserbehandlung/
[2] Hartinger Handbook of Wastewater and Recycling Technology, Günter Dietrich, Hanser Verlag
[3] Detoxification of nickel, copper and cyanides, Galvanotechnik, issue 9/2022


