3D printing is an integral part of medical technology and is constantly being developed further. New technological approaches make it possible, for example, to design new material properties in the smallest of spaces.
Researchers at KIT have produced miniaturized porous materials from photoresist using a novel photo-microprinting process (section 1). But even without new developments, additive manufacturing techniques are essential for medical technology, for example to bridge pandemic-related supply bottlenecks (section 2). To ensure uninterrupted printing, 3D printers are equipped with sensors such as a filament sensor. However, these are not sufficient for critical 3D prints - new sensor approaches are described in section 3.
Novel photoresist enables 3D printing of the smallest porous structures [1]
 Color change: The micro-cylinder on the right, printed with the novel photoresist, appears white because the light is scattered in its sponge-like structure, while the cylinder printed with conventional photoresist appears transparentIn theCluster of Excellence 3D Matter Made to Order (3DMM2O), scientists from the Karlsruhe Institute of Technology and Heidelberg University are conducting interdisciplinary research into innovative technologies and materials for digital scalable additive manufacturing processes in order to make 3D printing more precise, faster and more efficient[2]. The aim is to fully digitize 3D manufacturing and material processing from the molecule to the macrostructure. In addition to funding as a Cluster of Excellence within the Excellence Strategy of the German federal and state governments, 3DMM2O is also funded by the Carl Zeiss Foundation.
Color change: The micro-cylinder on the right, printed with the novel photoresist, appears white because the light is scattered in its sponge-like structure, while the cylinder printed with conventional photoresist appears transparentIn theCluster of Excellence 3D Matter Made to Order (3DMM2O), scientists from the Karlsruhe Institute of Technology and Heidelberg University are conducting interdisciplinary research into innovative technologies and materials for digital scalable additive manufacturing processes in order to make 3D printing more precise, faster and more efficient[2]. The aim is to fully digitize 3D manufacturing and material processing from the molecule to the macrostructure. In addition to funding as a Cluster of Excellence within the Excellence Strategy of the German federal and state governments, 3DMM2O is also funded by the Carl Zeiss Foundation.
A photoresist for two-photon microprinting has now been developed in this Cluster of Excellence, with which three-dimensional polymer microstructures with nano-sized cavities can be produced for the first time. In the journal Advanced Materials, the scientists from the joint Cluster of Excellence 3D Matter Made to Order report on how the porosity can be controlled in the printing process and how this affects the light scattering properties of the microstructures [3].
Photoresists are printing inks that can be used to 3D print the smallest microstructures in so-called two-photon lithography. During printing, a laser beam is moved through the initially liquid photoresist in all spatial directions. The photoresist only hardens at the focal point of the laser beam. Gradually, complex microstructures can be built up. In a second step, a solvent washes out the areas that have not been exposed. What remains are complex polymer architectures on a micro- and nanometer scale.
Two-photon polymerization - or rather two-photon microprinting based on this process - has been the subject of intensive research for several years, for example with regard to the production of micro-optics, so-called metamaterials or micro-scaffolds for experiments with individual biological cells. New printable materials are needed to expand the range of applications. This is where the scientists at the 3DMM2O Cluster of Excellence come in: With previous photoresists, it has only been possible to print transparent, glass-like polymers. The new photoresist makes it possible for the first time to print 3D microstructures from porous nanofoam. This polymer foam has cavities between 30 and 100 nanometers in size that are filled with air. This approach makes it possible for the first time to print "white" material with photoresist for 3D laser microprinting, because, as in a porous eggshell, the numerous tiny air holes in the porous nano-architectures cause them to appear white. Simply mixing white particles into a conventional coating would not be a solution, as the photoresist must be transparent to the (red) laser beam during printing. The resist is transparent before printing, but the printed objects are white and therefore highly reflective. The researchers from Karlsruhe and Heidelberg demonstrate this property by printing a hair-thin integrating sphere, a component used in technical optics.
Another factor that opens up new possibilities is the extremely large inner surface of the porous material. This could have a positive effect in filter processes in the smallest of spaces, in extremely water-repellent coatings or in the cultivation of biological cells.
What the new type of photoresist is suitable for and how it can be used in the best possible way has been described in an overarching collaboration between three of the nine research focuses of the Cluster of Excellence. Using electron microscope scans and optical experiments, the researchers showed how the cavities are distributed in printed structures and how their formation can also be controlled by changing the pressure settings, especially the strength of the laser pulses. Heidelberg researchers in the field of materials science and Karlsruhe researchers in the fields of chemistry and physics are involved in the current work in the Cluster of Excellence.
3D-printed medical products close supply bottlenecks in times of pandemic [4, 5]
When borders are closed to contain the spread of Covid-19, companies are forced to adapt their supply chains. Additive manufacturing facilities using 3D printers responded quickly by pooling resources and reducing pressure on supply chains. Among other things, the focus was on 3D printing technologies to close supply gaps. These existed primarily in nasal swabs, ventilator components and personal protective equipment. Additive manufacturing is currently also improving the supply of important products such as face shields, valves, filters, pressure sensors and X-ray tubes. Applications range from general care to high-precision and personalized devices - even for niche markets.
 Professional 3D printing system: In the research project, IPH uses the X500PRO industrial printer from German RepRap GmbH and equips it with sensor technology
Professional 3D printing system: In the research project, IPH uses the X500PRO industrial printer from German RepRap GmbH and equips it with sensor technology
Even before the pandemic, analysts predicted that the market for additive manufacturing in medicine would reach a value of at least 20 billion US dollars by 2020. In dentistry, the market is expected to grow by USD 9.7 billion by 2027, with an impressive annual increase of 35%.
A key advantage of additive manufacturing is the ability to close gaps in supply with timely serial production capacities. It also enables complex and functional designs to be produced from a single piece - without the need for subsequent assembly of individual parts. This often also increases the quality of the products. Added to this is the ability to produce prototypes cost-effectively and with shorter development times. The pandemic has not only made it clear that both are possible, but also how extensive the device-specific regulations and regulatory requirements are.
 3D-printed dental splints: Thanks to additive manufacturing, medical devices can be individually adapted to the patient's body and still be produced cost-effectively
3D-printed dental splints: Thanks to additive manufacturing, medical devices can be individually adapted to the patient's body and still be produced cost-effectively
Medical devices must be of high quality, efficient and safe. Numerous conformity and safety standards must be met before they can be placed on the market. The intended use determines further special requirements for the product. Personal protective equipment must keep particles, droplets and the like away from the user (Regulation EU 2016/425). The conformity and safety standards are particularly high for protective masks and face shields, such as those used in hospitals. The required conformity assessment takes time, which is in short supply during a pandemic.
Guidelines help to implement the regulatory requirements reliably and promptly. TÜV SÜD has developed checklists on the most important requirements from central standards and regulations for additive manufacturing and made them available free of charge during the crisis - both general and specific. This benefits testing laboratories, healthcare professionals and the general public. In addition, international standards organizations such as ASTM International and ISO provide free access to standards relevant to the manufacture and testing of personal protective equipment and medical devices.
In this way, additive manufacturing helps to combat the pandemic and has an overall positive impact on the medical and healthcare industry because it encourages innovation. There is much to suggest that fast, integrated supply chain networks with local production will become the new normal. As an independent third party, TÜV SÜD not only provides support with checklists. Its specially developed testing services for additive production facilities ensure the quality and consistency of industrial, additive series production. These help 3D printing contract manufacturers to ensure compliance with the requirements of the MDD and MDR.
Governments and industry associations, multinational companies and start-ups want to use platforms to close knowledge gaps in the industry:
Siemens made its 3D printers available to doctors, hospitals and manufacturers who required the development of medical devices or components. Siemens is also networking the entire value chain from design and simulation to production.
Singapore's AM accelerator National Additive Manufacturing Innovation Cluster (NAMIC) has set up a Covid-19 resource page that lists comprehensive resources for medical facilities, hospitals and medical device suppliers. They are working with 3D printing hubs to design, optimize and print parts for critical healthcare devices.
3Yourmind, an agile manufacturing software provider, created a platform to more efficiently coordinate and organize deliveries of products needed during the pandemic. The company integrated the TÜV SÜD checklist into its workflow so that the manufactured components could be evaluated in the process.
3D printer manufacturer Ultimaker is using the Covid-19 online portal to help healthcare centers find production capacities in their area. The company also helps with design, print preparation and post-processing as well as application. The first TÜV SÜD checklist was created together with Ultimaker.
The user-oriented network Mobility Goes Additive (MGA) supports its members in the development of medical products and the exchange of technical know-how. It collects application examples, links and FAQs on face shields, masks and respiratory devices. As a member, TÜV SÜD supports manufacturers in the implementation of regulatory requirements.
TÜV SÜD participated in a multi-agency collaboration in Singapore with the Health Science Authority, Nanyang Technological University (NTU) and NAMIC. The aim: to guide manufacturers through the testing requirements so that they can meet them reliably and quickly. Checklists for face shields and nose swabs are available free of charge via NAMIC's Covid-19 response platform.
The Centre for Additive Manufacturing at the National University of Singapore (AM.NUS) signed a Memorandum of Understanding (MoU) with TÜV SÜD last year. The MoU relates to research and development activities in additively manufactured biomedical metal implants for better healthcare. Their production requires ISO 13485 certification for a comprehensive quality management system for medical devices. TÜV SÜD contributes its expertise here with training courses.
Ensuring quality in 3D printing - with sensors and artificial intelligence [6]
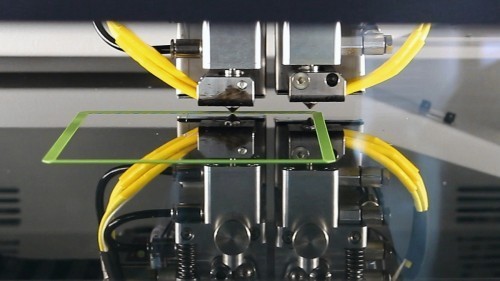 Quality monitoring: The scientists will attach sensors to the printer to detect errors such as a clogged print head3D printing enables production in batch size 1. This means that no two products are the same because each part is individually designed for the customer. This is interesting for medical technology, for example. Additively manufactured dental splints, hearing aids and even implants can be perfectly adapted to the body of an individual patient and still be produced cost-effectively. One challenge for companies, however, is quality assurance. Personalized medical devices are subject to strict safety requirements. Manufacturers must be able to guarantee that there are no invisible cracks or pores in the component and that the geometric requirements and desired material properties are achieved. Scientists from Hanover and Aachen are working on a solution to support small and medium-sized companies in particular with the approval of patient-specific medical devices from 3D printers: They want to develop an app that helps with quality assurance.
Quality monitoring: The scientists will attach sensors to the printer to detect errors such as a clogged print head3D printing enables production in batch size 1. This means that no two products are the same because each part is individually designed for the customer. This is interesting for medical technology, for example. Additively manufactured dental splints, hearing aids and even implants can be perfectly adapted to the body of an individual patient and still be produced cost-effectively. One challenge for companies, however, is quality assurance. Personalized medical devices are subject to strict safety requirements. Manufacturers must be able to guarantee that there are no invisible cracks or pores in the component and that the geometric requirements and desired material properties are achieved. Scientists from Hanover and Aachen are working on a solution to support small and medium-sized companies in particular with the approval of patient-specific medical devices from 3D printers: They want to develop an app that helps with quality assurance.
The more individualized the production, the more difficult the quality assurance. For goods that are manufactured in large series, it is often sufficient to carry out quality tests on a few randomly selected products in order to be able to draw conclusions about the entire batch. This is not possible with custom-made products. Here, each component must be examined in detail to ensure quality.
Identifying potential defects in patient-specific 3D printed products during production is the aim of a new research project being carried out jointly by scientists from the Institut für Integrierte Produktion Hannover (IPH) gGmbH and the Laboratory for Machine Tools and Production Engineering WZL at RWTH Aachen University [7].
The researchers want to equip an industrial 3D printer with sensor technology in order to seamlessly monitor the printing process. The sensor data will be evaluated in an app using artificial intelligence via a quality model in order to reliably detect production errors. The scientists at IPH and WZL are focusing primarily on medical technology because the degree of individualization of products and the associated quality requirements are particularly high in this industry and proof of a quality assurance system must be provided as part of approval. The IPH is responsible for sensor technology and data collection in the research project, while the WZL is responsible for creating the quality model and programming the app.
Quality in 3D printing is influenced by many factors: the type of material used, the ambient temperature, the temperature at which the filament is melted, the printing speed, the vibrations of the print head and a number of other parameters. Additive manufacturing processes react very sensitively to external influences. The aim is to achieve greater reliability in the process and avoid errors. It is particularly tricky that many errors are no longer visible from the outside once the component has been printed. For example, if the print head clogs for a short time and then continues to print normally, this cannot be seen later.
IPH is using the X500PRO industrial printer from German RepRap GmbH for the research project. The plastic used for printing is acrylonitrile butadiene styrene (ABS), which has a comparatively high strength but is very temperature-sensitive.
The scientists want to use various sensors to monitor the print quality. Possible options include sensors that measure the temperature of the build plate or build chamber, infrared sensors that can be used to determine the temperature directly at the print head, vibration sensors and optical measurement technology, which IPH has already investigated in the Quali3D research project [8].
Acoustic signals, in simple terms: sound recordings, are also informative for quality monitoring. These could be used, for example, to determine if the print head is clogged or the filament in the system breaks. This can be heard, but the challenge is to process the signals in such a way that ambient noise is filtered out - for example, the whirring of the motor in the 3D printer, the hissing of the computer or a slammed door. The researchers want to use machine learning, a form of artificial intelligence, to distinguish between important and unimportant noises. By training the program with as many sound recordings as possible, it learns better and better to reliably detect errors based on acoustic signals.
Ultimately, the aim is to develop an app that automatically evaluates all sensor data. The aim is to reduce the effort involved in quality assurance in 3D printing and also enable non-experts to monitor the print using an intuitive app. Users do not have to interpret the sensor data - the app monitors the entire printing process, documents errors and provides feedback on the print quality. In the event of serious errors that could render the component unusable, the app stops the printing process and informs the user. The print settings can then be adjusted and specialist staff can be called in if necessary.
Literature
[1] Source: Karlsruhe Institute of Technology (KIT) www.kit.edu/kit/pi_2020_077_neuartiger-fotolack-ermoglicht-3d-druck-kleinster-poroser-strukturen.php
[2] www.3dmm2o.de
[3] F. Mayer; D. Ryklin; I. Wacker; R. Curticean; M. Calkovský; A. Niemeyer; Z. Dong; P.A. Levkin; D. Gerthsen; R.R. Schröder; M. Wegener: 3D Two-Photon Microprinting of Nanoporous Architectures, Advanced Materials 2020, doi: 10.1002/adma.202002044
[4] Source: TÜV Süd
[5] More information at: www.tuvsud.com/en/industries/manufacturing/machinery-and-robotics/additive-manufacturing/industrial-additive-manufacturing-production-site-certification www.tuvsud.com/de-de/branchen/produzierende-industrie/maschinen-geraete-ausruestung/additive-fertigung/zertifizierte-unternehmen
[6] Source: IPH Institute for Integrated Production Hanover www.iph-hannover.de
[7] www.iph-hannover.de/de/dienstleistungen/fertigungsverfahren/additive-fertigung/
[8] www.iph-hannover.de/de/forschung/forschungsprojekte/?we_objectID=5424&pid=5805


