Researchers make progress in 3D printing of human tissue. Scientists from Munich and Würzburg are among the pioneers
Heart valves
In the human body, four heart valves ensure that the blood is directed in the right direction. It is crucial that the heart valves open and close correctly. To ensure this function, the heart valve tissue is heterogeneous, which means that the heart valves have different biomechanical properties within their structure.
A research team led by Petra Mela, Professor of Medical Engineering Materials and Implants at the Technical University of Munich (TUM), and Professor Elena De-Juan Pardo from the University of Western Australia, has now imitated this heterogeneous structure for the first time using a 3D printing process called melt electrowriting [1]. To this end, they have developed a manufacturing platform that enables the high-precision printing of individual patterns and combinations thereof. This enabled them to precisely customize various mechanical properties within the basic structure of a heart valve [2].
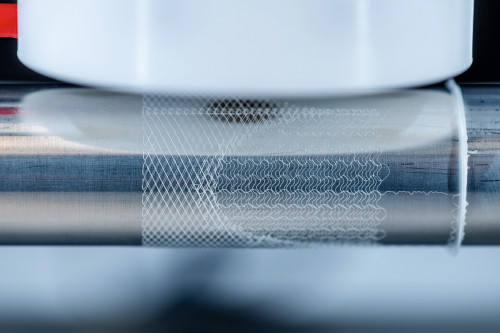 Melt electrowriting is a comparatively novel additive manufacturing process in which high voltage electricity is used to form precise patterns from a very thin polymer fiber. A polymer is heated, melted and pressed out of a print head as a liquid jet
Melt electrowriting is a comparatively novel additive manufacturing process in which high voltage electricity is used to form precise patterns from a very thin polymer fiber. A polymer is heated, melted and pressed out of a print head as a liquid jet
During the process, a high-voltage electric field is used to significantly reduce the diameter of the polymer jet by accelerating the jet and drawing it towards a collecting device. This creates a very thin fiber with a diameter typically in the range of five to fifty micrometers. The electric field also stabilizes the polymer beam. This is a prerequisite for creating clearly defined and precise patterns.
The "writing" of predefined patterns with the fiber beam is carried out using a computer-controlled collecting platform. This moving platform catches the emerging fiber in a clearly defined path, similar to a slice of bread being moved back and forth under a dripping spoonful of honey. This path is specified by the user by programming coordinates.
In order to significantly reduce the programming effort required to produce complex structures for heart valves, the scientists have developed software that makes it possible to assign individual patterns to different areas of the heart valve scaffold. These can be selected from a collection of available patterns. In addition, geometric specifications such as length, diameter and cross-section of the support scaffold can be easily customized via a graphical user interface.
The team used medically approved polycaprolactone (PCL) for 3D printing, as it is compatible with cells and biodegradable. The researchers are thus pursuing the concept that after implantation of the PCL heart valves, the patient's own cells will grow on the porous scaffold, potentially forming new tissue before the PCL structure degrades. Cell growth on the scaffold has already been observed in initial cell culture studies.
The PCL scaffold is embedded in an elastin-like material that mimics the properties of the body's own elastin in real heart valves. It also has micropores that are finer than those of the PCL scaffold. This is intended to leave enough space for the cells to colonize, but at the same time the valves are tight enough to ensure blood flow.
The 3D-printed heart valves were tested in an artificial circulatory system that simulates the body's own blood flow and pressure. Under the conditions tested, the heart valves opened and closed properly.
The PCL material was further developed and evaluated. By modifying the PCL with so-called ultra-small superparamagnetic iron oxide nanoparticles, the researchers were able to visualize the carrier scaffolds using magnetic resonance imaging (MRI) technology. Even with this modification, the material is still printable and compatible with body cells. This could facilitate the use of this technology in clinics, as the scaffolds can be visualized during implantation.
The aim is to create bioanalog heart valves that promote the formation of new functional tissue in the patient. Children in particular could benefit from such a solution, as currently available heart valves do not grow with the patient and therefore have to be replaced in several operations over the years. These heart valves, on the other hand, mimic the complexity of the body's own heart valves and are designed in such a way that they enable the patient's body cells to infiltrate the supporting scaffold.
The next step towards clinical application is preclinical studies in animal models. The team is also working on further improving the technology and developing new biomaterials.
Blood vessels
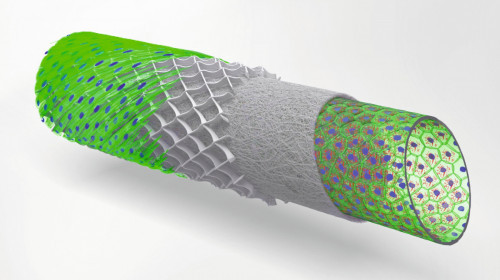 The schematic drawing shows a concept of multi-layered blood vessel models (Photo: UKW/Tomasz Jüngst)Dr. Tomasz Jüngst, Junior Professor of Bioprinting at the University of Würzburg, investigates blood vessels - from large veins to the smallest capillaries. The physicist has already developed numerous 3D bioprinting processes during and after his doctorate at the Institute for Functional Materials and Biofabrication at the University Hospital of Würzburg. The institute is one of the world's leading institutions in the processing of 3D bioprinting methods and materials.
The schematic drawing shows a concept of multi-layered blood vessel models (Photo: UKW/Tomasz Jüngst)Dr. Tomasz Jüngst, Junior Professor of Bioprinting at the University of Würzburg, investigates blood vessels - from large veins to the smallest capillaries. The physicist has already developed numerous 3D bioprinting processes during and after his doctorate at the Institute for Functional Materials and Biofabrication at the University Hospital of Würzburg. The institute is one of the world's leading institutions in the processing of 3D bioprinting methods and materials.
With new 3D bioprinting technologies, various cells, molecules and biomaterials can be hierarchically and spatially integrated into a matrix, from which artificial tissue can mature. Tomasz Jüngst specializes in blood vessels [4]. Tomasz Jüngst's team produces the scaffolding for blood vessel-like structures from a few "crumbs" of biopolymer, a type of plastic, in melt electrowriting systems that they have developed and built themselves. The work at the Institute for Functional Materials and Biofabrication, which is part of the University Hospital of Würzburg and Julius-Maximilians-Universität, ranges from large veins to the smallest capillaries. In the cell laboratory, various cell types are then placed in and on these sterilized tissue constructs. The first structures form in seven days and the cells are usually fully mature in 14 days. There is close cooperation here with the University of Utrecht in the Netherlands, and there are further collaborations with research groups in Brisbane and Sydney (Australia) and Christchurch (New Zealand). There are also joint projects on the local campus at Würzburg University Hospital.
In the EU-funded Design2Heal project, the Würzburg team studied cell-material interaction. Specifically, they investigated how the immune cell reacts to different fiber diameters and distances with the same material. With the self-built systems, materials can be printed that have a fiber size of 2 to 50 micrometers in diameter; in other processes, the fibers have a diameter of 200 to 400 micrometers. This interaction is currently also being used in an EU-funded project with the short name BRAVE(https://projectbrave.eu). The interaction between muscle cells and fibers is adapted in such a way that a biological "plaster" is created for the heart, whose function is impaired after a heart attack. The patch is intended to support the heart in maintaining its pumping capacity. Artificial hearts, known as VADs, are not a permanent solution. And donor organs are rare. However, the potential therapy method developed could be used in the longer term by using the patient's own cells and biomaterials, thus alleviating the problem of a lack of replacement organs.
If the polymers are combined with a hydrogel, different cell types can even be printed directly. The materials must be designed accordingly so that the cells accept the environment. The printing process takes place at 37 degrees and under sterile conditions. With his previous knowledge, he now wants to develop processes that are adapted to the properties of biofabrication. Cells must not only survive, they must not change during the printing process. A pluripotent stem cell, for example, i.e. a cell from a person's blood, bone marrow or skin, requires certain stimuli in order to develop. To ensure that the printing process does not provide it with a stimulus that goes in the wrong direction, it must be able to control the environment of the material as well as the mechanical forces, says Jüngst.
Although functioning organs will probably not exist for a long time yet, the researchers' aim is to create tissue models that can be used to test therapies in order to further reduce the number of animal experiments, for example. It is not possible to achieve the complexity of a body, but certain aspects can be imitated and reproduced. In this way, drugs can be tested in a standardized way, but the medication and dose can also be individually adapted to the patient because the cells can be taken directly from the patient. The organ-on-a-chip method, for example, in which organs can be reproduced using cell clusters and several organs can be assembled, is very promising. Such pioneering approaches make it possible to study the interaction of organs and the effects of drugs on individual organs.
Literature
[1] Source TUM
[2] Saidy, N.T.; Fernández-Colino, A.; Heidari, B.S.; Kent, R.; Vernon, M.; Bas, O.; Mulderrig, S.; Lubig, A.; Rodríguez-Cabello, J.C.; Doyle, B.; Hutmacher, D.W.; De Juan-Pardo, E.M.; Mela, P.: Spatially Heterogeneous Tubular Scaffolds for In Situ Heart Valve Tissue Engineering Using Melt Electrowriting, Adv. Funct. Mater. 2022, 2110716, doi.org/10.1002/adfm.202110716
[3] Mueller, K.M.A.; Topping, G.J.; Schwaminger, S.P.; Zou, Y.; Rojas-González, D.M.; De Juan-Pardo, E.M.; Berensmeier, S.; Schilling, F.; Mela, P.: Visualization of USPIO-labeled melt-electrowritten scaffolds by non-invasive magnetic resonance imaging, Biomater. Sci. 2021, 9, 4607-4612 doi.org/10.1039/D1BM00461A
[4] https://www.med.uni-wuerzburg.de


