Implants for the facial area enable the surgical correction of deformities following accidents and tumor removal as well as due to age-related bone loss. Currently, plastic implants are used that are not adapted to the patient's anatomy and are only adjusted during the operation. Often inadequate anchoring to the bone entails risks of displacement and thus disfigurement as well as bacterial infection risks. The development goal for temperature-sensitive, 3D-printable polymers was therefore antimicrobial coatings for optimal bone growth using atmospheric pressure plasma spraying of hydroxyapatite (HAp). Part 1 of a two-part series.
Facial implants are widely used for the surgical correction of cosmetic, traumatic and congenital asymmetries, mostly on the nasal bridge, chin and cheek/jaw area - e.g. for the correction of disease-related malformations, loss of bone after tumor removal, accidental fractures and age-related loss of bone and tissue volume. The main aim of the treatment is to achieve an aesthetic result that is acceptable to the patient. Although autologous tissues are the gold standard from a biocompatible point of view, they require a second surgical site for tissue harvesting and carry the risk of tissue morbidity at both sites, can be resorbed by the surrounding bone and are difficult to sculpt into the desired shape. Therefore, alloplastic, synthetic materials are predominantly implanted to replace missing bone volume, whereby these must be biocompatible, free of significant foreign body reactions, stable enough to withstand physiological stresses (forces during speech, chewing and facial expressions) and easy to shape preoperatively or intraoperatively to model the contour [1]. Common implant materials in facial surgery [2-4] are titanium alloys [5] for high mechanical requirements, e.g. in the jaw, and polymers such as expanded polytetrafluoroethylene (ePTFE, GoreTex) [6], polyethylene terephthalate (PET) [7], polyethylene ((HD-)PE) [8], polypropylene (PP) [9] and polydimethyl silicone [10] for lower expected loads. Bone cements made of PMMA and bioactive hydroxyapatite [11] that are molded in situ during surgery are also used, although these have poor predictability of the result due to in-situ molding. PE and silicone are predominantly used due to their good moldability. Porous materials (PE, ePTFE) enable better tissue integration (ingrowth of bone cells), but this is problematic if implant removal is necessary due to significant tissue damage to the soft tissue and underlying bone and fragmentation of the implant.
Incorrect placement or fixation Incorrect placement or fixation with subsequent migration/rotation of the implant and visible contour changes is the most common complication (up to 15 % of cases) [12, 13] - migration/rotation often occurs with a significant time delay and - like the frequent selection of an incorrect implant size - leads to excessive stress on the surrounding tissue and thus to neuropraxia (malfunction of nerves due to overstretching), ischemia, necrosis or extrusion (perfusion of the overlying skin), edema and ecchymosis. The consequences are chronic pain stimuli and visible hematomas and seromas (abnormal fluid accumulations). The latter support bacterial and later biofilm growth (thick layers of bacteria) and trigger bacterial inflammation of the soft tissue and bone close to the implant (peri-implantitis) [1]. Most infections in the early postoperative period occur with porous implants with large surfaces [2]. Infections that occur years after surgery, regardless of implant porosity, are due to hematogenous spread of bacteria (from the dental area [14]) or direct injury to the tissue encapsulation that has formed around the bioinert implant. Explanations are necessary in the case of strong inflammatory reactions, but also in the case of bone resorption as a result of excessive pressure loading of non-anatomically adapted implants
Patient-specific implants from generative manufacturing - process and availability of biocompatible (polymer) materials
Based on cranio- / maxillofacial tumor surgery with severe facial deformities, generative manufacturing (AM, "3D printing") of implants is also an optimal method for general plastic surgery in the facial area in order to avoid a large number of the complications mentioned above - especially due to the perfect adaptability to the patient-specific anatomy of the existing bones. Based on computer tomography (CT) data, 3D scaffolds with very complex contours are created virtually, which requires not only commercial software packages from Materialise (Mimics, 3Matic), 3Dsystems (RapidForm, Geomagic) and Vision Imaging (Amira) but also a high level of anatomical-functional and surgical experience for (re)construction with the necessary high degree of accuracy [15, 16]. Subsequently, layer-by-layer fabrication is performed directly from a computer-aided design file by extrusion (e.g. fused deposition modelling, FDM), polymerization (stereolithography), selective laser melting and sintering (SLM, SLS) or direct writing processes [17-23].
Bioresorbability with complete replacement of the implant by the body's own bone tissue is not desirable in the field of facial surgery due to the fact that the esthetic appearance cannot be planned in detail in advance and may still change postoperatively. However, bioactivity of the surface to avoid complications is of great importance. PEEK and various photopolymers, for example, are bioinert and biocompatible at the same time. However, due to its high melting temperature, PEEK is very difficult to produce using SLS and FDM, whereas 3D printing of photopolymers, e.g. using the PolyJet process, is technically simpler, cheaper and faster. In this process, the liquid monomer is applied to a work platform by a print head with several nozzles and immediately cured by UV light. The platform then moves down by the thickness of one layer (16-32 µm) and another layer is applied on top of the already cured layer. This process is repeated until the model is completely printed. Droplet-shaped application makes it possible to melt before the curing process, which means that the grooves in the workpiece known from SLS and FDM are not visible and a smooth surface is formed. However, the flowing process requires support structures for overhanging areas, which must be removed after production [24].
While bioinert (facial) implants printed using the PolyJet process fulfill the requirements for high long-term patient-specific accuracy, requirements regarding osteointegration to prevent rotation and migration of the implants as well as antimicrobial protection must be achieved by subsequent bioactive surface modification.
State of the art: Concepts for improving osteointegration and preventing bacterial infections of implanted biomaterials
Bioinert plastics and titanium alloys are predominantly used for implants due to their strength, absence of tissue toxicity (cytotoxicity) and resistance to biodegradation/corrosion. However, to avoid the formation of fibrous tissue capsules around the implants and thus inflammation or implant migration, long-term implantation requires surface properties, e.g. bone cements or coatings made of materials very similar to the mineral components in the bone, i.e. calcium phosphates such as hydroxyapatite (HAp) [25, 26]. In recent decades, various coating processes for HAp coatings (i.e., plasma/thermal spraying, magnetron sputtering and ion-assisted vacuum coating, dip coating, sol-gel coating, electrophoresis) have been developed specifically for metallic implants (hip/knee endoprostheses) [27].
Plasma and thermal spraying are currently used almost exclusively on metal substrates in industrial applications. Poor adhesion of the coatings [28] and high (post) treatment temperatures to achieve the necessary phases are the disadvantages of almost all processes. For temperature-sensitive plastic substrates (i.e. with the exception of PEEK [29-31]), no usable/adhesive coatings are currently available. However, excellent coating adhesion [32] is essential, which is mainly controlled by intrinsic residual stresses due to cooling and bioresorption by the Ca-P phases present at the interface.
Experimental - Atmospheric pressure plasma spray coating
In atmospheric pressure plasma nozzles (plasma jets), a pulsed arc is generally generated by means of a high-voltage discharge (5-15 kV, 10-100 Hz), past which the process gas flows and is ionized. The outlet is point-shaped through a nozzle head as a thermal hot gas plasma, which is at ground potential and thus largely retains potential-carrying parts of the plasma flow. In particular, the internal structure of the plasma nozzle and the excitation voltage and frequency used define the achievable plasma properties (density, energy, etc.). The decisive know-how in the material development described below is a patented hot gas plasma nozzle from Inocon Technologie GmbH (InoCoat IC 3, Fig. 1), which has emerged from the Plasmatron plasma welding technology and which, compared to competitors, operates stably at a much lower power and thus over a significantly larger power range, which significantly improves the controllability of plasma processes (patents EP0933982, EP0962277). Special know-how on electrode and nozzle shape (including simulation methods) and gas flows enables significantly broader lateral homogeneity of the plasma. This extends the plasma residence and reaction times of particles, gases and precursors with an optimized feed point and angle (part of Inocon patent DE1021014100385).
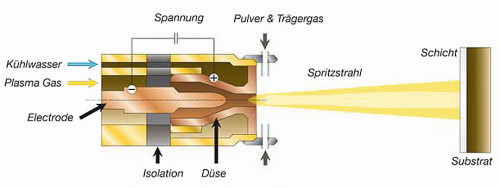 Fig. 1: Schematic diagram of a hot gas plasma nozzle from Inocon Technologie GmbH
Fig. 1: Schematic diagram of a hot gas plasma nozzle from Inocon Technologie GmbH
The advantage of atmospheric pressure plasma coating is the universal usability of gaseous and vaporous precursors for the deposition of SiO2 [33-35], TiO2 [36, 37] and polymer thin films (see overview in [35]) as well as the simple and also combined usability with thick film processes for metal, oxide and polymer layers using microparticles or sprayed-on liquid precursors (atmospheric pressure plasma spraying). The stoichiometry of complex ceramic compounds (such as HAp) can be transferred comparatively easily into coatings by using powders in the atmospheric pressure plasma spraying process, similar to plasma or thermal spray processes. So far, however, only very few publications and patents have dealt with these APPD plasmas, which can be controlled very well in terms of their plasma energy and over much larger ranges than spray processes, for the production of ceramic coatings on polymers or in combination with thin-film processes to optimize adhesion (i.e. for HAp with plasma jets generally only on high-temperature-resistant PEEK plastic substrates [38]).
In the course of the development work, two different HAp powder types (Ca5(PO4)3OH) with existing medical approval from MediCoat (Mägenwil, Switzerland) were used, which differ in particle size (63±15 µm and 25±5 µm) but not in chemical composition.
Based on experience, the IC3 plasma jet from Inocon Technologie GmbH enables the coating of very temperature-sensitive substrates. A urethane acrylate (exo-1,7,7-trimethyl-bicyclo(2.2.1)-hept-2-YL acrylate) from Stratasys was used as a biocompatible material with tests according to ISO 10993 and already available medical approval as an implant material for short-term use (up to 28 days), whereby smooth and hexagonally microstructured flat samples were printed using PolyJet technology [39]. This polymer with 65 MPa tensile strength, >20 % elongation at break, < 3 GPa modulus of elasticity (ASTM D638), 30 J/m Izod impact strength (ASTM 256) serves as the basis for the developments, which can thus also be used for future, permanently implantable and 3D printable implant materials as well as for typical materials already in use today, such as polyether ether ketone (PEEK) and polyether ketone ketone (PEKK). In addition, complex overhanging and porous structures can be printed using support material that can be completely removed in a water bath.
Detailed descriptions of the characterization methods used can be found in the following section of the presentation of the research results.
Results and discussion
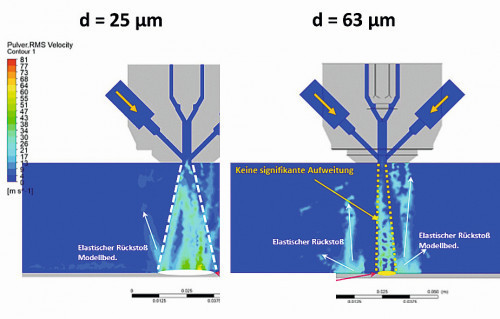 Fig. 2: Process modeling with digital twin for powder selection: Comparison of particle speed, temperature and melted particle content for 25 and 63 µm powder
Fig. 2: Process modeling with digital twin for powder selection: Comparison of particle speed, temperature and melted particle content for 25 and 63 µm powder
The first step in coating development was to simulate the processes in the plasma as a function of different powder particle sizes of the hydroxyapatite using the digital twin for the Inocon IC3 plasma jet, which is based on a computational fluid dynamics simulation validated for a large number of powders and precursors (Ansys Software 2019 R3, CFD). This allows useful findings to be obtained in advance with regard to particle flight speed, particle melting behavior and particle distribution. Available material properties of MediCoat's HAp powders served as a database alongside the typical plasma jet parameters. The results of the discussion - including the backscattering behavior as a basis for estimating the resulting overspray - are shown in Figure 2, where the wider coating cone of the finer powder can be seen very clearly. The evaluation as a Gaussian distribution makes it clear that the particle velocity spectrum at the outlet of the plasma nozzle for the finer HAp powder (25 µm) after the plasma nozzle is broader than that of the coarser HAp powder (63 µm). The average particle velocity of the fine HAp powder is about 3 times higher than that of the coarser HAp powder. Similar behavior can be predicted for the particle temperatures achieved (i.e. without taking into account temperature gradients in the particle due to slow heat conduction): The mean particle temperature of the fine HAp powder amounts to about 700 K and that of the coarser HAp powder to about 350 K. Consequently, the Gaussian distribution for the mean liquid phase fraction of the finer HAp powder shows only very low values (i.e. only very few particles are predominantly melted), while no liquid fraction can be achieved with the coarser HAp powder. These results were successfully validated experimentally via the coating characteristics (e.g. powder weight fraction deposited as a layer vs. overspray) with an average relative deviation range of 20 %.
Based on a comprehensive parameter study on the adjustable process parameters for atmospheric pressure plasma spraying (power of the plasma jet, plasma and powder feed gas flows, powder flow, distances, movement speeds, etc.), stable process parameters were found which can be used to optimize the coating process.Stable process parameters were found which do not lead to substrate damage either optically (deformation, discoloration after degradation) or in detailed surface analysis of the selected substrate polymer in Fourier-transformed infrared spectroscopy (FTIR/ATR mode after mechanical layer removal). Results from scratch tests of coatings up to 100 µm thick also show very good adhesion properties without extensive delamination in the vicinity of the scratches.
Subsequently, for these selected parameters of sufficiently high heat input into the particles, but only low heat input into the substrate surface via the plasma itself, the coating formation characteristics will be discussed as a function of the heat input. According to the state of the art for these biomaterials, this in particular has a significant effect on the subsequent biocompatible behavior (i.e. osteointegration) via the phase formation.
![Abb. 3: Links: Vorgänge beim der Abscheidung (Plasma-Spritzbeschichtung) eines teilweise aufgeschmolzenen HAp-Partikels, rechts: auftretende Phasen und Löslichkeiten (log KSP) beim Spritzen von HAp (nach [44]) Abb. 3: Links: Vorgänge beim der Abscheidung (Plasma-Spritzbeschichtung) eines teilweise aufgeschmolzenen HAp-Partikels, rechts: auftretende Phasen und Löslichkeiten (log KSP) beim Spritzen von HAp (nach [44])](/images/stories/thumbnails/thumb_gt-2023-01-034.jpg) Fig. 3: Left: Processes during the deposition (plasma spray coating) of a partially melted HAp particle, right: phases and solubilities (log KSP) occurring during the spraying of HAp (after [44])
Fig. 3: Left: Processes during the deposition (plasma spray coating) of a partially melted HAp particle, right: phases and solubilities (log KSP) occurring during the spraying of HAp (after [44])
It is known from the literature that crystalline HAp starting powders can disintegrate during spray coating (plasma spraying, thermal spraying) due to the influence of temperature to form CaO, amorphous calcium phosphate and α-TCP (tricalcium phosphate), all of which have significantly higher solubility in body fluids (or physiological saline solution) and/or hydrolyze quickly, which has a negative effect on adhesion to the surrounding bone after implantation during the ingrowth phase [40]. Crystalline HAp with very low solubility would be the optimal state (table in Fig. 3). In addition, the specific volume increases and initiates cohesive and adhesive cracks in the porous coatings. β-TCP (through phase transition of α-TCP) dissolves or hydrolyzes much more slowly, while crystalline c-HAp (HA in Fig. 3) is the most stable [41]. Stable c-HAp should therefore be formed for highly bioactive, well-adhering coatings. Small amounts of more rapidly resorbable phases support the formation of new bone. If the HAp melts strongly in the plasma during thermal or plasma spraying, high cooling rates after splat deposition on the substrate surface suppress crystallization to c-HAp almost completely (Fig. 3). Only repeated temperature treatment, as is the case as a result of several passes during plasma spraying, which greatly increases the temperature of the substrate over longer periods of time (minutes), increases the c-HAp content [42, 43]. However, this is not possible for polymers, but also for warpage-sensitive, finely structured e.g. TiAl6V4 substrates. The heat deflection temperature (HDT) for the selected urethane-acrylate substrate, for example, is 40-45 °C, while the glass transition temperature is 50-55 °C. This is very low compared to other currently used technical polymers with implant use (e.g. PEEK), but typical for many biocompatible and resorbable future medical technology polymers (e.g. medically approved polycaprolactane with slow degradation).


