The Covid pandemic has also brought with it a surge of innovation in diagnostics. Within a very short time, reliable detection methods had to be developed, countless laboratories had to be equipped and several hundred thousand tests had to be carried out every week [1]. Two microfluidic systems that can be used for SARS-CoV-2 detection are presented below.
SARS-CoV-2 detection in 30 minutes with gene scissors [2]
The so-called CRISPR-Cas gene scissors are versatile: apart from the popularly known 'gene editing' for the production of the controversially discussed genetically modified organisms (GMOs for short), the effector protein Cas in particular is now also used in different variants in a large number of studies for the molecular biological detection of nucleic acids such as DNA or RNA.
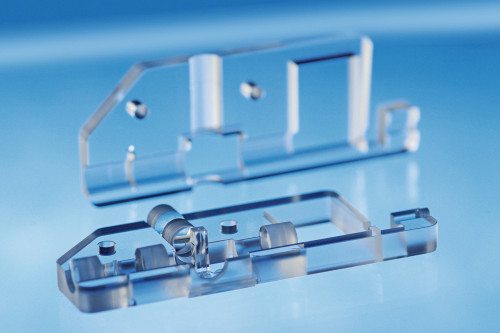
In its latest study, the Freiburg research team led by microsystems engineer Dr. Can Dincer from the Institute of Microsystems Engineering presents a microfluidic multiplex chip on which the viral load in the nasal swab and, if necessary, the antibiotic concentration in the blood of Covid-19 patients could be measured simultaneously [3].
The development of rapid tests for the detection of SARS-CoV-2-specific antigens has had a significant impact on how society deals with the pandemic: instead of having to wait one to three days for an appointment for an rt-qPCR test, which is not always easily available, rapid tests are now relatively conveniently available in every drugstore, pharmacy and even supermarket. However, what is saved in costs, effort and time with the latter is paid for in the form of test sensitivity. This problem was particularly evident last winter, when infections with the Omikron variant of the virus were detected very late and often only after the onset of symptoms by the so-called 'lateral flow devices'. "The trade-off between test sensitivity and sample-to-result time could be bridged with our method," says Midori Johnston, first author of the study, which now appears in the journal Materials Today [3] (Fig. 1).
As with the rapid test at home or in test centers, a patient sample (nasopharyngeal or oropharyngeal swab) is dripped into a reaction mix. In contrast to the conventional rapid test, however, CRISPR does not test for viral proteins, but for characteristic sequences in the viral genome, as in the PCR test. If the sample contains the desired RNA fragment, the effector protein (Cas13a) is activated and cuts the reporter RNA present in the reaction solution. The absence of this reporter results in an inversely proportional relationship between the amount of viral RNA from the sample and the measured current density when the chip is read out electrochemically. The system does not require amplification of the genetic material, is flexibly adaptable to new, infectiologically relevant mutations of the virus and uses only inexpensive, durable and non-toxic reagents as well as a handy measurement setup.
In light of the recent decisions by several federal states to abolish the isolation requirement for patients who have tested positive for Covid-19, reliable, sensitive and rapid testing options are once again necessary in order to be able to react appropriately to renewed waves of infection. Hospitalization with severe courses will also be inevitable. This is where another feature of the microfluidic chip comes into play: the combination of the CRISPR assay with ß-lactam antibiotic detection. People suffering from Covid-19 often also become infected with bacteria and are treated accordingly with broad-spectrum antibiotics such as amoxicillin, ampicillin or piperacillin. The right dose is crucial to ensure successful treatment, but also to reduce the development of resistant germs. The research group's sensor could be useful here by simultaneously monitoring both the viral load and the antibiotic concentration in the patient's blood or saliva.
Other researchers from the University of Freiburg were involved in this interdisciplinary study: Prof. Dr. Wilfried Weber from the Chair of Synthetic Biology and CIBSS - Centre for Integrative Biological Signalling Studies, Dr. med. Daniela Huzly from the Institute of Virology at the University Medical Center Freiburg and Prof. Dr. Gerald Urban from the Chair of Sensors at the Institute of Microsystems Engineering (IMTEK).
Multiplex analysis with paramagnetic microparticles [1]
A team at the Fraunhofer Institute for Laser Technology ILT in Aachen has developed a new readout unit for paramagnetic particles in a microfluidic system [1].
The special feature here is the type of particle: They are microparticles of different sizes and different fluorescences, which are "loaded" with different capture molecules (antigens or antibodies) as required, so that up to 24 analytes can be captured simultaneously (Fig. 2).
 Fig. 2: Microfluidic readout unit for subsequent clinical multiplex analysis (Photos: Fraunhofer ILT, Aachen)
Fig. 2: Microfluidic readout unit for subsequent clinical multiplex analysis (Photos: Fraunhofer ILT, Aachen)
Many questions have arisen from this: How can tests be scaled up more quickly in the future, how can different tests be carried out simultaneously? The experts at Fraunhofer ILT and Institut Virion\Serion GmbH have tackled this last question in particular in a joint project. They have developed a combination of assay and readout unit that will allow a large number of different tests to be carried out simultaneously in future (Fig. 3).
"A normal flow cytometer is designed for cells," explains Dr. Georg Meineke from Fraunhofer ILT. "We have developed a system for analyzing microparticles, which we can detect simultaneously across three sizes and different fluorescence levels in 24 different channels." The different species of these particles can be clearly identified using scattered light and fluorescence measurements.
For the actual diagnostics, each particle type can be provided with a specific capture molecule that precisely binds an analyte to be detected. The bound analyte molecules are then detected using a fluorescent secondary marker. In this way, many different diagnostic markers and, in the case of antibody detection, even up to three immunoglobulin classes can be detected simultaneously in a single process step.
While the project partner Institut Virion\Serion GmbH focused on the particles and the appropriate assay, the researchers at Fraunhofer ILT developed the corresponding microfluidic readout unit. It was trimmed to a compact design and real-time data processing.
The project partners have built a functional model for a subsequent in-vitro diagnostic device that automatically measures particle samples and their associated analytes. The subsequent in-vitro diagnostic device currently allows the detection of up to 24 different individual markers with the additional option of reading up to three different secondary markers in parallel. The electronic platform developed for this purpose controls the measurement system in real time and records the measurement data. It enables integration into automation solutions, such as a fully automatic laboratory machine.
Literature
[1] Source : Source : Fraunhofer ILS
[2] Source: Albert-Ludwigs-Universität Freiburg - FIT Freiburg Center for Interactive Materials and Bioinspired Technologies & Institute for Microsystems Engineering - IMTEK
[3] Johnston M.; Ates H.C.; Glatz R.; Mohsenin H.; Schmachtenberg R.; Göppert N.; Huzly D.; Urban G.A.; Weber W.; Dincer C.: Multiplexed biosensor for point-of-care Covid-19 monitoring: CRISPR-powered unamplified RNA diagnostics and protein-based therapeutic drug management, in: Materials Today, 2022, DOI: 10.1016/j.mattod.2022.11.001