Modern microscopes for biological imaging are often "black boxes" whose exact operating principle remains unknown and whose optical resolution and price seem to be inversely proportional to each other. UC2 (You. See. Too.) presents a low-cost, 3D-printed, open-source, modular microscopy kit and demonstrates its versatility. A complete microscope development cycle from concept to experimental phase is realized.
Introduction
The brightfield microscope, enclosed in an incubator, monitors the differentiation of monocyte to macrophage cells at cellular resolution level (e.g. 2 μm) for seven days. In addition, the geometry is transferred to a 400-euro light sheet fluorescence microscope for volumetric observations of a transgenic zebrafish expressing green fluorescent protein (GFP) by incorporating very few additional components. The aim is to establish an open standard in optics to facilitate coupling with various complementary platforms. By making the content and comprehensive documentation publicly available, the systems presented here are suitable for simple and straightforward replications, modifications and extensions. The growing demand in biological research for spatial and temporal resolution, imaging volume, molecular specificity and high throughput is leading to increasingly complex and expensive microscopes [1, 2]. In addition to numerous imaging techniques, long-term observations of living organisms, with minimal impact on their natural behavior, have become an essential aspect of light microscopy. The need to keep the cells in a well-controlled environment poses additional constraints, which are addressed by imaging within an incubator [3, 4] or by using incubator units on microscopes [5-8]. Assembling, maintaining and improving microscopes, as well as analyzing and verifying the generated data, very often requires a consulting specialist to deal with the particular device, further widening the gap between microscope engineers and users [1, 2]. While customized solutions are commercially available for a variety of imaging tasks, such as those mentioned above, they are often costly, difficult to extend or modify, and rarely sufficiently documented for users to adapt them for "out-of-the-box tasks" outside their primary intended use.
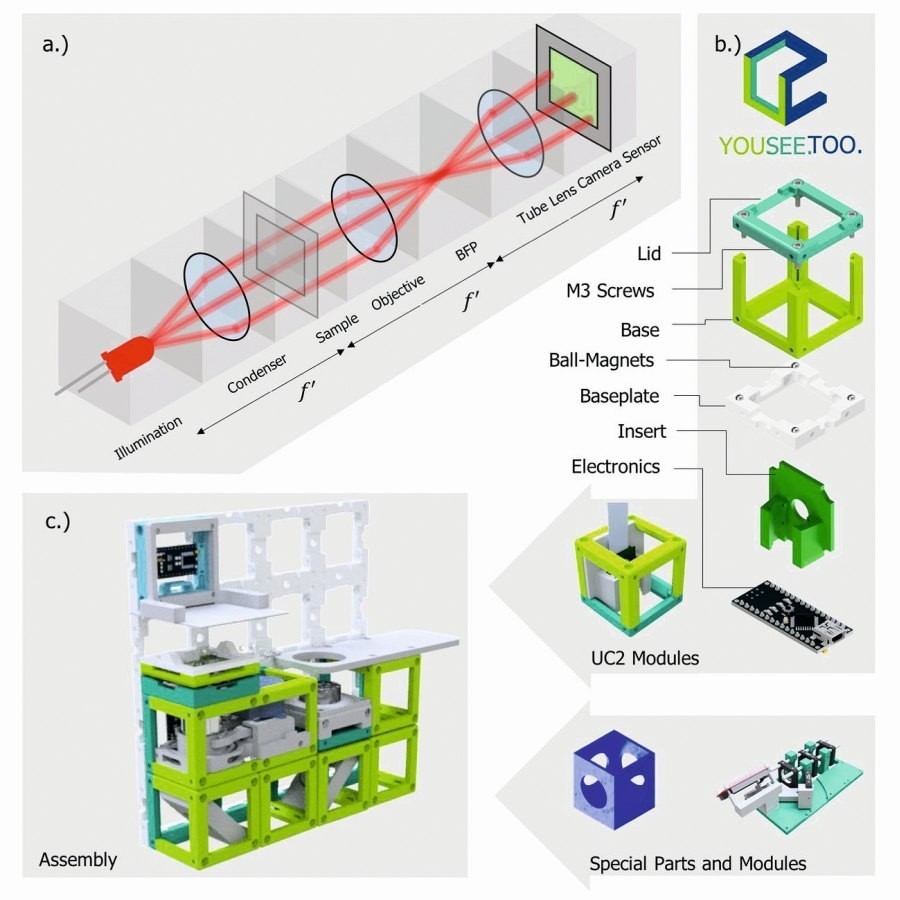 Fig. 1: a) The 4f system divides Fourier optical arrangements into functional units, where f' corresponds to focal lengths. BFP corresponds to the back focal plane (i.e. the pupil plane). b) The unit element (cube) serves as the basic framework for any component that fits inside (lens, camera, Z-autofocusing mechanism, etc.). b) A magnetic snap mechanism connects the optical building blocks into a skeleton to realize mechanical stability and rapid prototyping of a given optical setup. c) An exemplary setup of a microscope for an ordinary smartphone (not shown) and an inexpensive lens as a combination of available modules. The cubes fit on the base plate grid in the design grid of 50 × 50 mm2 (see Supplementary Notes 3).
Fig. 1: a) The 4f system divides Fourier optical arrangements into functional units, where f' corresponds to focal lengths. BFP corresponds to the back focal plane (i.e. the pupil plane). b) The unit element (cube) serves as the basic framework for any component that fits inside (lens, camera, Z-autofocusing mechanism, etc.). b) A magnetic snap mechanism connects the optical building blocks into a skeleton to realize mechanical stability and rapid prototyping of a given optical setup. c) An exemplary setup of a microscope for an ordinary smartphone (not shown) and an inexpensive lens as a combination of available modules. The cubes fit on the base plate grid in the design grid of 50 × 50 mm2 (see Supplementary Notes 3).
Regardless, science is approaching a reproducibility and quality crisis given the growing pressure to publish as quickly as possible [11]. Open research, where every step is transparently recorded and made fully accessible to the public, can help to restore confidence in the scientific literature, which has been visibly compromised in recent years [12].
Modern optical setups are reaching immense complexity as they combine a growing number of optical and photomechanical components. They usually originate from different manufacturers who adhere to various industry standards such as the International Organization for Standardization (ISO) or the Royal Microscopy Society (RMS), whose intracompatibility is often not guaranteed. This makes it particularly difficult to customize or even reconfigure optical systems, requiring handmade adapters or unnecessarily long attachments that compromise the integrity and stability of the systems.
What can be seen as a significant room for improvement is an open standard [13] that enables a simple interface between the components of modern microscopes such as sources, optics, optomechanics and detector components. Such a platform would enable simple designs of versatile imaging instruments that can be easily adapted to almost any imaging task. Switching from one imaging system to another could be reduced to a mere reconfiguration rather than a new design. Such a tool would not only be useful for research, but also immensely helpful in optics teaching. It would significantly reduce the effort required to build a system and allow students to actively perform system reconfigurations within minutes. Such a hands-on experience would lead to a fundamental understanding and allow anyone to view optics as a playground where many ideas can be easily explored. To realize such a system, an open standard is crucial, as this is the only way to allow effortless conversion without limiting the possibilities too much. Fortunately, many major steps have already been taken in this direction.
Recent approaches such as the Flamingo [9, 10] aim to establish light sheet microscopy as a service for everyone and thus address the problem of lack of accessibility. A number of very well documented open source projects such as the Lattice Light Sheet [14] or openSPIM [15] lead to educational workshops and thus encourage users to contribute to their development. In terms of hardware design, projects such as the "open-flexure stage" [16], the "100-euro lab" [17], the smartphone-based "Foldscope" [18] and open-source systems for single-molecule localization microscopy (SMLM) [19, 20] demonstrate flexible and cost-effective microscopy solutions with high performance. More generic approaches have been realized in the form of an opto-mechanical building block [21] and in the form of a functional box-like unit called μCube [22]. With the widespread availability of and easy access to rapid prototyping tools such as 3D printing, programmable electronics (e.g. Arduino [23]), high-quality cameras in smartphones or mini-computers (Raspberry Pi [24]), it is now actually possible to develop an open standard that is accessible to everyone and thus ensures wide dissemination, adaptation and expansion. Impairments in image quality due to less corrected, inexpensive optical components or less stable mechanical arrangements can often be compensated for in real time by intelligent electronics and software algorithms. Methods such as autofocus routines, deconvolution [25] or the recovery of hidden information such as the quantitative phase with simple LED arrays [26] are current examples of such possibilities.
The UC2 (You. See. Too) approach aims to create such open standards. By using the concept of matching focal planes (Fig. 1a), UC2 is particularly easy to use, flexibly reconfigurable and versatile for a wide range of applications. It is equipped with open source software, open design files and blueprints for a wide variety of configurations and openly accessible documentation. UC2 offers students at all levels a cost- and time-efficient way to experience the design and application of a variety of complex optical setups. Furthermore, it provides a broad group of users and developers with access to modern light microscopy by utilizing purely commercially available, consumer-grade components (Supplementary Notes 1 and 8 for the parts list) to produce low-cost microscopic imaging devices for approximately 100 to 400 Euros.
The manuscript describes the entire development cycle of a brightfield microscope integrated into an incubator from its assembly to its successful application using four identical systems for a parallel 168 h in vitro imaging session from monocytes to macrophages. The device will also be converted into a light sheet microscope using the original brightfield microscope assembly and only a few additional components. To demonstrate the applicability of UC2 in biomedical research, imaging results will be provided from a variety of biological samples, including fluorescent transgenic human microvascular lung endothelial cells, Drosophila melanogaster, zebrafish, E. coli bacteria, obtained using a range of UC2-based microscope modes, in particular brightfield, widefield fluorescence, image scanning microscopy, intensity diffraction tomography and structured illumination.
Results Open standard: the basic cube
Modern microscopes with infinity-corrected objectives are often set up in the so-called 4f configuration (Fig. 1a), in which the lenses are arranged so that the focal planes (f) of adjacent elements coincide in order to limit the amount of optical imaging errors, realize telecentricity and predict the system behavior using Fourier optics [27]. The name 4f is derived from the sum of the focal lengths of a simple imaging system with two neighboring lenses that are constructed in such a way that the focal planes lie on top of each other, resulting in 2f per lens and thus 4f in total. Here, this fundamentally modular principle is implemented in the form of a general 3D-printed frame in which individual modules (i.e. optical building blocks) are arranged in cube form (Fig. 1b and Supplementary Notes 2) such that the focal planes of optics in successive cubes often coincide.
By analyzing many available optical components, imaging systems and frames, it was found that a design grid of dblock = 50 mm seems to offer an optimal balance between compatibility, handling and flexibility for enabling Fourier optical (4f) setups. The division of the cube into a base and a lid simplifies printing with standard Fused Deposition Modeling (FDM) 3D printers and allows easy insertion of components as plug-ins.
Neodymium sphere magnets (Ø magnet = 5 mm), which are mounted in a grid shape on an extendable base plate, and ferromagnetic cylindrical stud screws (DIN 912), which sit in the edges of the cube, enable stable and precise magnetic attachment. Different orientations of the base plates enable construction in three dimensions. A four-point fixation was found to be a good compromise between the usual rectangular arrangement of the optical superstructures and mechanical stability.
External electrical and optical components (e.g. lenses, mirrors, LEDs; see Fig. 1b) and existing devices (e.g. rail systems from Thorlabs, Quioptics, Edmund Optics) can be easily adapted using plug-in and modifiable inserts (see supplementary notes 4). A Module Developer Kit (MDK, Supplementary Notes 1) with a generic reference design for customized inserts provides an easy interface for adding designs to the toolbox, even for users without technical training.
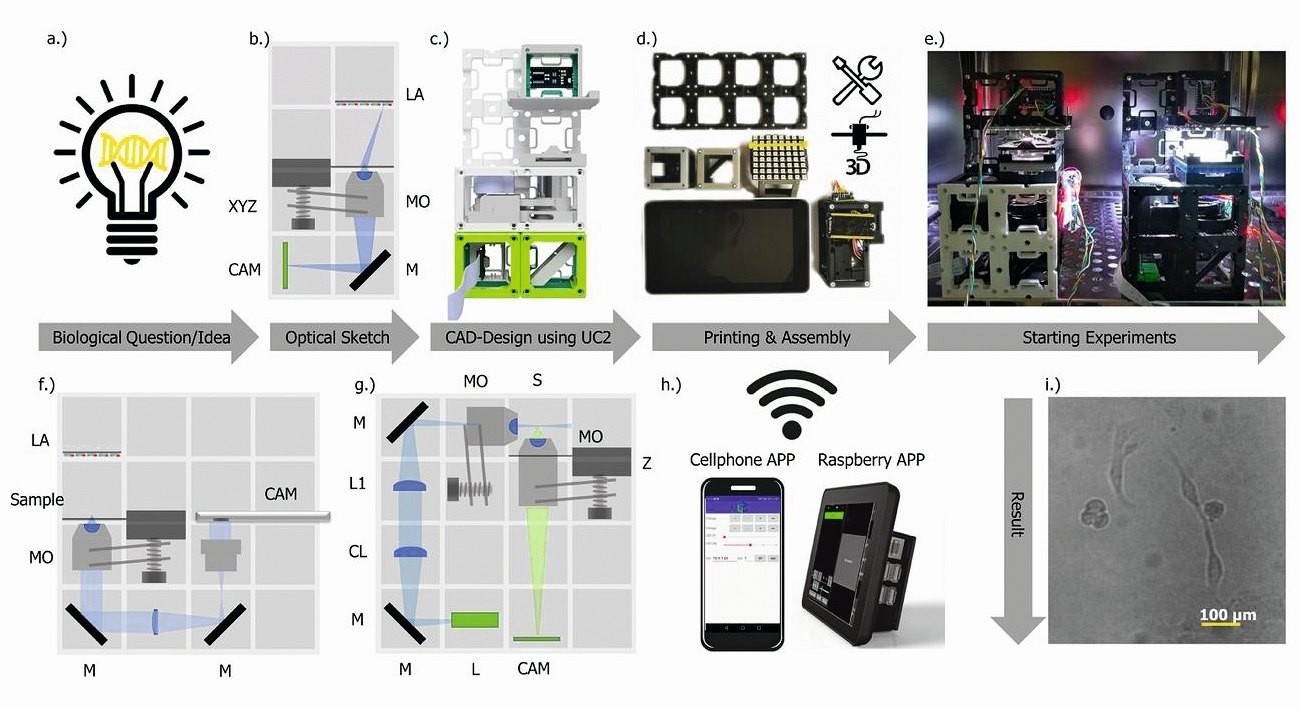 Fig. 2: Rapid prototyping with UC2. Typical workflow for creating a UC2 application: (a) Starting with a biological question/idea that requires an imaging device, which is designed in (b) (inverted incubator microscope) and realized in (c) with UC2 components from the CAD library. After printing and assembly (d), the device is placed in its working environment (e.g. incubator) (e) and is ready to record long-term image series of e.g. MDCK cells, which are visualized in (i) and in the supplementary video 6.
Fig. 2: Rapid prototyping with UC2. Typical workflow for creating a UC2 application: (a) Starting with a biological question/idea that requires an imaging device, which is designed in (b) (inverted incubator microscope) and realized in (c) with UC2 components from the CAD library. After printing and assembly (d), the device is placed in its working environment (e.g. incubator) (e) and is ready to record long-term image series of e.g. MDCK cells, which are visualized in (i) and in the supplementary video 6.
Remote control is achieved via "smart components" (e.g. cell phone, Raspberry Pi) in (h). The reuse of components allows conversion into a cell phone microscope (f) or light sheet microscope (g) within minutes (see Supplementary Video 5) and Supplementary Notes 7.8). CL: Cylindrical lens, TL: Tube lens, L: Laser, LA: LED array, M: Mirror, MO: Microscope objective, P-CAM: Detector (smartphone or Raspberry Pi), S: Sample positioning stage, F: Emission filter, Z: Focusing stage
The construction of increasingly complex optical systems from a simple magnifying glass to a fully functional fluorescence light sheet microscope (Fig. 2) is ensured by the library of modules based on this basic principle, which are combined and placed in the appropriate order (supplementary notes 5). The use of more complex commercially available components (cameras, motors, video projects, etc.) enables the use as a smart microscope and a remote control. Microcontrollers allow a wired (i.e. I2C28) or wireless (i.e. WiFi, IoT-based protocol using Message Queuing Telemetry Transport (MQTT) [29]) communication interface to control light settings or focusing mechanisms (Supplementary Notes 6.1). Power is supplied via the conductive magnets or wires with rectifiers in the cubes.
THE AUTHORS
Benedict Diederich 1, 2, 3, René Lachmann 1
Swen Carlstedt 4, Barbora Marsikova 1, 3
Haoran Wang 1, Xavier Uwurukundo 1
Alexander S. Mosig 4, Rainer Heintzmann 1, 2, 3
1 Leibniz Institute of Photonic Technology, Jena
2 Institute of Physical Chemistry and Abbe Center of Photonics, Jena
3 Faculty of Physics and Astronomy, Jena
4 Jena University Hospital, Institute of Biochemistry II, Jena
[1] Hell, S.W. et al: The 2015 super-resolution microscopy roadmap, J. Phys. D: Appl. Phys. 48, 2015, 443001
[2] Weigert, M. et al: Content-aware image restoration: pushing the limits of fluorescence microscopy, Nat. Methods 15, 2018, 1090-1097
[3] Kahle, J. et al: Applications of a compact, easy-to-use inverted fluorescence microscope, Am. Lab. 43, 2011, 11-14
[4] Kim, J.; Henley, B.M.; Kim, C.H.; Lester, H.A.; Yang, C.: Incubator embedded cell culture imaging system (EmSight) based on Fourier ptychographic microscopy, Biomedical Optics Express 7, 2016, 3097
[5] Lukinavidius, G. et al: Fluorogenic probes for live-cell imaging of the cytoskeleton, Nat. Methods 11, 2014, 731-733
[6] Frigault, M.M.; Lacoste, J.; Swift, J.L.; Brown, C.M.: Live-cell microscopy-tips and tools, J. Cell Sci. 122, 2009, 753-767
[7] Walzik, M.P. et al: A portable low-cost long-term live-cell imaging platform for biomedical research and education, Biosens. Bioelectron. 64, 2014, 639-649
[8] Hernández Vera, R.; Schwan, E.; Fatsis-Kavalopoulos, N.; Kreuger, J.A.; Modular and Affordable Time-Lapse Imaging and Incubation System Based on 3D-Printed Parts, a Smartphone, and Off-The-Shelf Electronics, PLoS ONE 11(Dec), 2016, e0167583
[9] Huisken, J.; Power, R.; Bakken, T.; Li, J.; Weber, M.: Flamingo Lightsheet https://involv3d.org/concept/,2019
[10] Power, R.M.; Huisken, J.: Putting advanced microscopy in the hands of biologists. Nat. Methods 16, 2019, 1069-1073
[11] Baker, M.: 1,500 scientists lift the lid on reproducibility, https://www.nature.com/news/1-500-scientists-lift-the-lid-on-reproducibility-1.19970
[12] Fanelli, D.: Is science really facing a reproducibility crisis, and do we need it to? Proc. Natl Acad. 115, 2018, 2628-2631
[13] Faber, M.J.: Open Innovation Approach by Chesbrough, 21-44 https://doi.org/10.1007/978-3-83498027-4{\_} 3. https://doi.org/10.1007/978-3-8349-80274_3 (Gabler, Wiesbaden, 2009).
[14] Chen, B.-C. et al.: Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution, Science 346, 2014, 1257998-1257998
[15] Pitrone, P.G. et al: OpenSPIM: An open-access light-sheet microscopy platform, Nat. Methods 10, 2013, 598-599
[16] Sharkey, J.P.; Foo, D.C.W.; Kabla, A.; Baumberg, J.J.; Bowman, R.W.: A one-piece 3D printed flexure translation stage for open-source microscopy, Rev. Sci. Instrum. 87, https://doi.org/10.1063/1.4941068, 2016
[17] Maia Chagas, A.; Prieto-Godino, L.L.; Arrenberg, A.B.; Baden, T.: The D100 lab: A 3D-printable open-source platform for fluorescence microscopy, optogenetics, and accurate temperature control during behavior of zebrafish, Drosophila, and Caenorhabditis elegans, PLoS Biol. 15, 2017, e2002702
[18] Cybulski, J.S.; Clements, J.; Prakash, M.: Foldscope: Origami-Based Paper Microscope, PLoS ONE 9, June 2014, e98781
[19] Martens, K.J.A. et al: Visualization of dCas9 target search in vivo using an open-microscopy framework, Nature Communications 10, 2019, 3552
[20] Diederich, B.; Then, P.; Jügler, A.; Förster, R.; Heintzmann, R.: cellSTORMCosteffective super-resolution on a cellphone using dSTORM, PLoS ONE 14, 2019, e0209827
[21] Winters, B.J.; Shepler, D.: 3D printable optomechanical cage system with enclosure. HardwareX 3, 2018, 62-81
[22] Delmans, M.; Haseloff, J.: µ Cube: A Framework for 3D Printable Optomechanics, J. Open Hardware 2, 2018, 1-9
[23] Arduino, I.: Arduinio-open source products for electronic projects, http:// www.arduino.org/ 2019
[24] Inc, R. Raspberry Pi -Teach, Learn, and Make with Raspberry Pi. https://www.raspberrypi.org/ 2016
[25] Booth, M.J.: Adaptive optical microscopy: the ongoing quest for a perfect image. Light: Science & Applications 3, 2014, e165-e165
[26] Tian, L.; Waller, L.: Quantitative differential phase contrast imaging in an LED array microscope, Opt. Express 23, 2015, 11394
[27] Gross, H.; Singer, W.; Totzeck, M.; Gross, H.: Handbook of Optical Systems, 2006, 1-690, https://doi.org/10.1002/3527606688
[28] Semiconductors, N. UM10204 I 2 C-bus specification and user manual Rev. 64 April 2014 User manual Documen is a machine-to-machine (M2M) Internet of Things http://www.nxp.com.
[29] For the Advancement of Structured Information, S. O. MQTT https://mqtt.org/ (2019).
[30] Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A.: Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes, Trends Immunol. 23, 2002, 549-555
[31] Guilliams, M.; Scott, C.L.: Does niche competition determine the origin of tissue-resident macrophages? Nature Reviews Immunology, https://doi.org/10.1038/nri.2017.42, 2017
[32] Andreesen, R.; Picht, J.; Löhr, G.W.: Primary cultures of human blood-born macrophages grown on hydrophobic teflon membranes, J. Immunol. Methods 56, 1983, 295-304
[33] Jay, S.M.; Skokos, E.; Laiwalla, F.; Krady, M.M.; Kyriakides, T.R.: Foreign body giant cell formation is preceded by lamellipodia formation and can be attenuated by inhibition of Rac1 activation, Am. J. Pathol. 171, 2007, 632-640
[34] Waldo, S.W. et al: Heterogeneity of human macrophages in culture and in atherosclerotic plaques, Am. J. Pathol. 172, 2008, 1112-1126
[35] McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape, Proc. Natl Acad. Sci. USA 110, 2013, 17253-17258
[36] Rosania, G.R.; Swanson, J.A.: Microtubules can modulate pseudopod activity from a distance inside macrophages, Cell Motil. Cytoskeleton. 34, 1996, 230-245
[37] Xia, Z.; Triffitt, J.T.: A review on macrophage responses to biomaterials, Biomed. Mater. 1, https://doi.org/10.1088/1748-6041/1/1/R01 2006
[38] Banterle, N.; Bui, K.H.; Lemke, E.A.; Beck, M.: Fourier ring correlation as a resolution criterion for super-resolution microscopy, J. Struct. Biol. 183, 2013, 363367
[39] Müller, C.B.; Enderlein, J.: Image Scanning Microscopy, Phys. Rev. Lett. 104, 12010, 98101
[40] Heintzmann, R.; Benedetti, P.A.: High-resolution image reconstruction in fluorescence microscopy with patterned excitation, Appl. Opt. 45, 2006, 5037-5045
[41] Diederich, B.; Wartmann, R.; Schadwinkel, H.; Heintzmann, R.: Using machine-learning to optimize phase contrast in a low-cost cellphone microscope. PLoS ONE 13, 2018, e0192937
[42] Ou, X.; Horstmeyer, R.; Zheng, G.; Yang, C.: High numerical aperture Fourier ptychography: principle, implementation and characterization, Opt. Express 23, 2015, 5473-5480
[43] Li, J. et al: High-speed in vitro intensity diffraction tomography, Advanced Photonics 1, 2019, 1-13