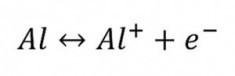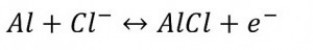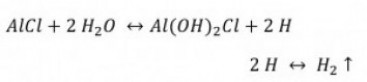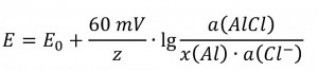Part 1: Basic reactions make the base metal technically useful
Solid but lightweight aluminum is becoming increasingly important as a lightweight component in aircraft and vehicle construction. However, only a thin oxide layer makes the base metal technically usable.
Aluminum is a very base metal (E0 = -1.66 V). It would therefore be of little technical use because it reacts violently even with water. Aluminum is a valve metal, i.e. it is a metal that forms a dense layer of a non-conductive metal oxide during anodic oxidation. It is the oxide layer that ensures technical usability. However, the natural oxide layer is very thin, around 4 nm. This oxide layer grows slightly over time (Fig. 1).
Oxide layer becomes thicker
As can be seen from the decreasing current densities in Figure 1, the oxide layer becomes slightly thicker with storage time. This dependency is shown again in Figure 2 .
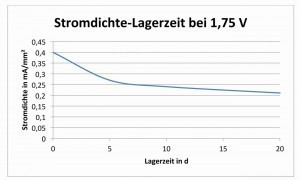 Fig. 2: Current density under condensation conditions with a voltage of 1.75 V applied as a function of the storage time in days
Fig. 2: Current density under condensation conditions with a voltage of 1.75 V applied as a function of the storage time in days
If one assumes that the voltage and the conductivity of the layer do not change, Ohm's law for the layer thickness follows:
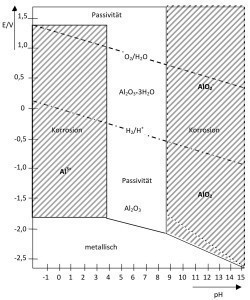 Fig. 3: Simple potential-pH diagram of aluminumInthe course of twenty days, the layer thickness approximately doubles.
Fig. 3: Simple potential-pH diagram of aluminumInthe course of twenty days, the layer thickness approximately doubles.
Unfortunately, it is also the case that passive layers on metals are always attacked - at least slightly. The passive layer of aluminum is only relatively stable in the pH range of around 4 to 9 (Fig. 3). At lower and higher pH values, it is severely attacked and can no longer guarantee protection. Stains for aluminum must therefore work in these ranges. However, as most applications are in the medium pH range, technical use is relatively unproblematic (Fig. 3).
Dense protective layers form on aluminum in the pH range of 4-10, which prevent corrosion. As expected, lowering the pH in the acidic range leads to an increase in the corrosion rate (Fig. 4).
The increase in corrosion rate with pH in the alkaline range is due to complexation. Al3+ ions do not dissolve, but [Al(OH)4] ions do.Another form of attack is by chloride.
Unfortunately, the oceans contain around three percent chloride. As a result, chloride is practically omnipresent and thus a major component of aluminum corrosion. This is why (filiform) corrosion is particularly pronounced near the sea
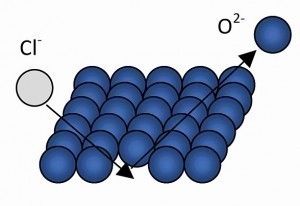
Pitting corrosion is the most common type of corrosion of aluminum and is initiated, for example, when an oxygen atom in the oxide layer is replaced by a chlorine atom (Fig. 5).
Self-healing through oxygen
This makes the layer, which is only a few atomic layers thick, more permeable at this point. The layer is impermeable because it was able to grow just enough to become dense.
With the further replacement of oxygen ions by chloride ions, white corrosion efflorescence (hydrolyzed aluminium chloride) develops locally. In the best case scenario, the oxygen displaces the chloride again and self-healing takes place, i.e. the pores "suffocate" and the corrosion (almost) comes to a standstill (Fig. 6 and 7).
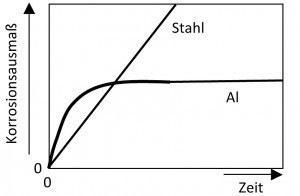
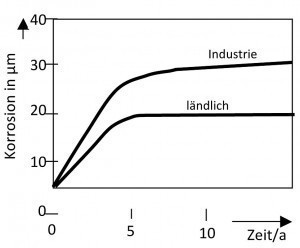
Pitting is also caused by the environment (Fig. 7). In industrial and sea air, corrosion is more severe than in land air. These environments contain chloride and other aggressive substances.
The composition and structure of the aluminum alloy is the second main factor influencing the corrosion of aluminum. For example, an alloy that is resistant to seawater (e.g. containing Mg; top layer: MgO) can fail completely in an acidic industrial atmosphere.
Less noble alloy partners dissolve
Pure aluminum naturally has the highest corrosion resistance. It decreases as the concentration of other metals in the alloy increases. Copper, with its high positive potential, has the strongest effect. In contrast, the more electronegative metals magnesium, zinc and chromium have a much smaller influence (Fig. 8).
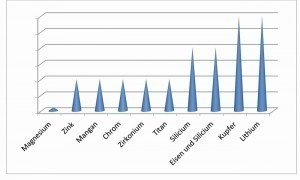 Fig. 8: Influence of the alloy components on corrosion behavior
Fig. 8: Influence of the alloy components on corrosion behavior
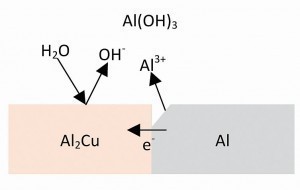 Fig. 9: Local element on a precipitate
Fig. 9: Local element on a precipitate
Contacts with foreign metals naturally also lead to potential formation. Some of these are listed in Table 1.
| Metal |
Potential against the |
Potential against |
| magnesium | -2372 | -850 |
| Zinc | -769 | -300 |
| AlZn 4 Mg 1 | -719 | -250 |
| AlZn 1 | -619 | -150 |
| AlMg 5 | -520 | -50 - -40 |
| AlMg3 | -520 | -30 - -20 |
| AlZnMgCu | -490 | -20 - -10 |
| AlMgMn | -480 | -10 - ±0 |
|
Pure aluminum |
-470 | ±0 |
| AlMgMn | -470 | ±0 - +10 |
| AlMn | -460 | +10 - +20 |
| Cadmium | -465 | ±0 - +20 |
| EN-WC AlSi 12 | -420 - -450 | +30 - +60 |
| EN-WC AlCu 4 | -370 | +100 |
| iron | -370 | +100 |
|
AlCuMg, |
-300 - -320 | +150 - +180 |
| Brass (50 % Zn) | -70 | +400 |
| nickel | +11 | +480 |
| copper | +80 | +550 |
|
Chrome-nickel |
-100 | +850 |
As a valve metal, aluminum protects itself with its dense oxide layer. However, it is only 4-5 nm thick, i.e. only a few atomic layers. Its resistance is somewhat lower at surface inhomogeneities, so that a low current flow is possible at these points. This leads to attack at these points. There are inhomogeneities at roughnesses, inclusions and roll-ins. However, they can also occur when individual atoms are detached from the bond (see above). The latter naturally occurs from time to time due to the attack of the surrounding medium, especially at high or low pH values of the corrosion medium. Such defects have been detected electrochemically [3, 4] and by electron microscopy [5].
Hydration energy determines ion stability
Hydrated Al3+ is always [Al(H2O)6]3+. This means that the inner water shell is chemically hydrated (stoichiometric composition). The outer shell is then predominantly physically bound (Coulombic forces). These hexaaquaaluminum ions act as a weak acid (pKs = 4.97) [8]. The level of hydration energy ultimately determines whether an ion is stable or not.
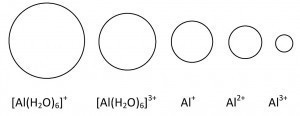 Fig. 10: Size ratios of the aluminum ions
Fig. 10: Size ratios of the aluminum ions
| ion | z |
r0 |
n |
-∆Gh0 |
-∆Hh0 |
-∆Sh0 |
ri |
z2/r |
| - | e | nm | - | kJ/mol | kJ/mol | J/(mol-K) | nm |
nm-1 |
|
Al+ |
1 | 6 | 464 | 485 | 70,7 | 0,129 | 7,752 | |
|
Al2+ |
2 | 1941 | 2021 | 270,7 | 0,107 | 37,383 | ||
|
Al3+ |
3 | 0,057 | 6 | 4577 | 4707 | 441,0 | 0,085 | 105,882 |
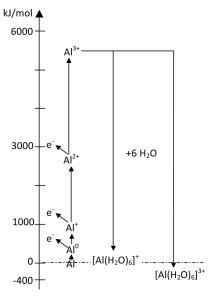 Fig. 11: Energy diagram of the formation of aluminum ionsThermodynamicdata of the hydration of aluminum ions at infinite dilution are summarized in Table 2. The determining parameter for the free energy is z2/r (Fig. 10). The energy ratios of the formation of aluminum ions are shown in Figure 11 (but without the extremely short-lived Al2+). As you can undoubtedly see, energy is only released in the trivalent ions. It is therefore the most stable of the aluminum ions.
Fig. 11: Energy diagram of the formation of aluminum ionsThermodynamicdata of the hydration of aluminum ions at infinite dilution are summarized in Table 2. The determining parameter for the free energy is z2/r (Fig. 10). The energy ratios of the formation of aluminum ions are shown in Figure 11 (but without the extremely short-lived Al2+). As you can undoubtedly see, energy is only released in the trivalent ions. It is therefore the most stable of the aluminum ions.
The unstable monovalent ions are stabilized by complexation. Chloride is an important stabilizer. The hydrated aluminium ions also hydrolyse. The coordination number 6 results in the following equilibrium for [Al(H2O)6]3+:
As a result, the corrosion electrolyte becomes more acidic. The cathodic reaction is:
in relation to the dissolved oxygen.
If corrosion products precipitate, the electrolyte is so highly concentrated that the water activity must also be taken into account.
It is difficult to explain the mechanism of aluminum dissolution because several reactions take place in parallel. The formation of Al3+ ions not only takes place via permeation reactions, but also via various chemical reactions (Fig. 12).
1. the disproportionation (DPR) of the Al+ ions formed
2. the reaction with hydrogen ions and
3. the reaction with water.
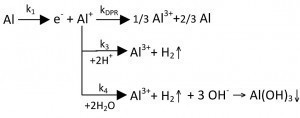 GT10-20_alu-gl-3.jpg GT10-20_alu-gr-12.jpg Fig. 12: Reaction diagram of the anodic reactions of aluminum
GT10-20_alu-gl-3.jpg GT10-20_alu-gr-12.jpg Fig. 12: Reaction diagram of the anodic reactions of aluminum
Effect of the chloride
Chloride is pure poison for aluminum, even in small doses. Why?
A through reaction such as Al ↔ Al3+ + 3 e- will never take place in this way because 4 particles will never collide or fly apart at the same time. Such a summation reaction must therefore take place in stages. The first stage is then:
In the case of aluminum, this is the detachment of the 3p1electron, the two 3s electrons are bound much more tightly. The Al+ ion is now relatively unstable and short-lived. It continues to react immediately (Fig. 12).
In acidic solution with the hydrogen ionsAl++ 2 H+ ↔ Al3++ H2and in alkaline solution with the hydrogen ions of water Al++ 2 H2O↔ Al3++H2+ 2 OH- .
If chloride is present, it stabilizes the Al+ ion. But that's not all, it also catalyzes the permeation reaction of the aluminium. Protective, stable reaction products are formed during the reaction with water. As a result, the corrosion site heals itself. This is not the case with the permeation reaction involving chloride.
If chloride is present, this reaction is preferred, as it takes place at a more negative potential than without.
The aluminum chloride continues to react as long as free water is present.
Dissociation of the basic salt:
The resulting hydrogen partially escapes to the outside as a gas. However, as it is initially formed atomically, the other part diffuses into the aluminum and leads to local embrittlement, which can have an accelerating effect on stress corrosion cracking.
With dissociation, the chloride is available again for the next passage reaction. Chloride therefore acts as a catalyst for aluminum dissolution. Corrosion can progress. The consequences are, for example, pitting corrosion and filiform corrosion.
The potential of the pitting reaction depends on the chloride concentration with 60 mV per order of magnitude (1 Cl- and 1 e-) [6, 7]
Conclusion for the electroplater from the mode of action of the chloride: If corrosion problems occur, city water (chlorinated!) should never be used as the final rinsing water.
Literature
[1] Füssel, U., project leader: Correlation between surface history, process conditions and the soldering suitability of aluminum materials; SB IGF 17.748 BR, TU Dresden (2015)
[2] Pryor, M.J.; Keir, D.S.: J. Electrochem. Soc. 105(1957), p. 629
[3] Wood, G.C. et al.: Proc. Conf. on Localized Corrosion 1971 Williamsburg, NACE Houston (1974)
[4] Galvele, J.R.: J. Corr. Sci 28(1981), p. 351
[5] Thompson, G.E. et al: J. Electrochem. Soc. 129(1982), p. 1515
[6] Kaesche, H.: Die Korrosion der Metalle, 3rd ed. (2011) Springer Verlag
[7] Kosin, L.F.: Electrodeposition and dissolution of polyvalent metals (Russian)
[8] Holleman, A.F.; Wiberg, N.: Lehrbuch der anorganischen Chemie, 102nd edition, De Gruyter, Berlin (2007)


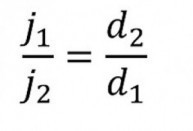
![pH-Abhängigkeit des Masseverlustes min g/(m2*d) von Reinstaluminium (Al 99,999) in lufthaltiger NaCl-Lösung c(NaCl) = 1 mol/L nach [2] pH-Abhängigkeit des Masseverlustes min g/(m2*d) von Reinstaluminium (Al 99,999) in lufthaltiger NaCl-Lösung c(NaCl) = 1 mol/L nach [2]](/images/thumbnails/thumb_gt-2020-10-0016.jpg)