Chromium stands for the multicolored nature of its salts and its many uses in the surface industry. Whether we take a shower in the morning, check our emails on our smartphone or get into the car, hexavalent chromium is used everywhere to create certain surface properties.
Legal basis for chromium trioxide (EU and CH)
Hexavalent chromium is assessed as a substance of very high concern (SVHC). According to the REACh Regulation (Registration, Evaluation, Authorization and Restriction of Chemicals), it may only be used with a time-limited authorization from the ECHA (European Chemicals Agency). In order to avoid any trade barriers, Switzerland has amended its Chemicals Ordinance and included chromium trioxide in the list of prohibited substances in Annex 1.17 of the Swiss Chemical Risk Reduction Ordinance (ChemRRV). If no hexavalent chromium remains in the end product, these processes can continue to be used in Switzerland and consumption only has to be reported to the Federal Office of Public Health in Bern. If hexavalent chromium remains in the end product (e.g. chromating), it may only be used if an identical EU authorization is available.
No chromic acid remains on the goods in electrolytic deposition processes with bright and hard chrome plating as well as in ablative processes such as pickling and etching of metals and plastics. Chromium trioxide remains on the end product in conversion layers (chromating) and oxide layers (anodizing).
Example of substitution using bright chrome deposition
In bright chromium plating, it is technically possible to replace chromium trioxide (chromic acid) with chromium (III) electrolytes. A disadvantage is the lower bluish-cold color of deposited chromium layers from Cr3+ ions. Compliance with customer requirements is a key aspect of substitution. The list of requirements often includes color, corrosion resistance, abrasion resistance, etc. These can only be determined through lengthy trials and tests with the new electrolyte.
Investment in plant technology
Switching to a different chemical substance also requires a change in plant technology with corresponding investments and, above all, production interruptions during the conversion. The trivalent electrolyte is operated with mixed oxide-coated titanium stretched metal anodes instead of lead anodes. Plastic tanks, filter devices and pipelines can no longer be used as residues of chromic acid have diffused into the equipment. The investment required to replace the equipment, including an ion exchanger system for removing iron for bath maintenance, "cannot be licked away" (... nothing can be done about it).
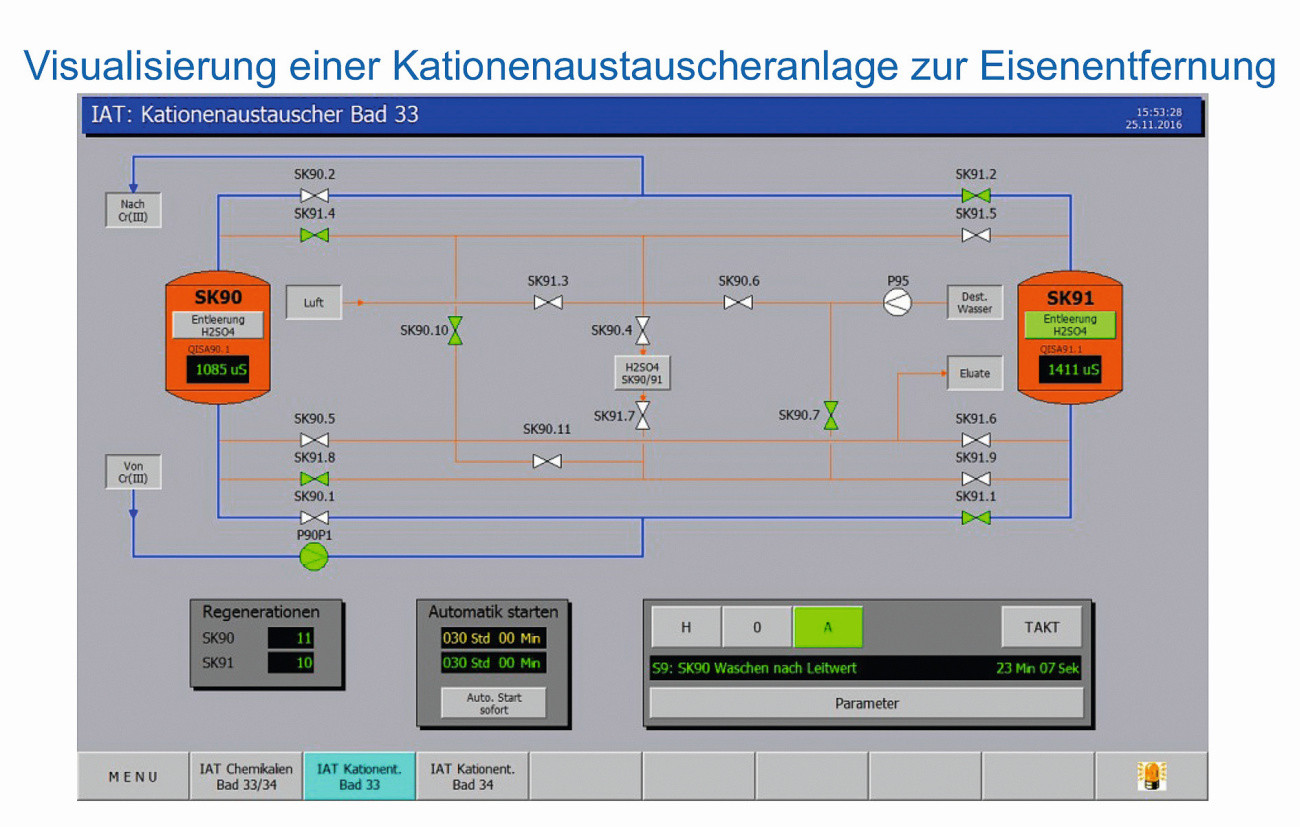 Visualization of a cation exchanger system for iron removal (Photo: ProWaTech AG)
Visualization of a cation exchanger system for iron removal (Photo: ProWaTech AG)
In a specific example from 2016, the investment amounted to approx. 205,000 euros including installation for a volume of 9,000 l of chrome electrolyte and up to 750m2 of product throughput per day.
Analytical effort
While a hexavalent electrolyte usually requires a weekly check of chromate (Cr6+), sulphate (SO42-) and, if necessary, analyses of foreign metals such as Cr3+ and Fe, trivalent electrolytes usually require daily checks of metals (nickel, iron, chromium), pH value and boric acid. In addition, at high production rates, the bath additives must be measured weekly by HPLC analysis. This also applies to other metals (depending on the base material), and a Hull cell test is also necessary.
Disadvantages of chromium (III)
An identical color compared to chromium (VI) electrolytes is often required in order to be able to mix the goods deposited from chromium (VI) and chromium (III). However, this is rarely achieved. The lower layer thicknesses are also a disadvantage. With hexavalent bright chromium electrolytes, 1-3 µm of chromium can be deposited, thus increasing the abrasion resistance, e.g. for electrical plugs.
Due to the very high boric acid concentration in the process bath of trivalent electrolytes, the boric acid salt is passed through a filter system during preparation and subsequent dosing. A minimum temperature of the process bath must be ensured at all times, otherwise crystallization will occur.
Advantages of chromium (III)
According to users, trivalent chromium solutions for bright chrome plating are up to 15 times more expensive to purchase and for bath management (analysis) than previous hexavalent process solutions. However, if the total costs are considered, these are only around 1.1 times (+ 10 %) the costs. There are significant savings with complex surface geometries due to higher throwing power, which leads to a reduction in reworking of the workpieces. Burning in the high current density range and lack of deposition in the low current density range (holes) are more pronounced with chromium (VI) electrolytes. As a result, defects and reworking were reduced by approx. 95 % after substitution in a practical example.
The reduction of hexavalent chromium is no longer necessary in chemical wastewater treatment. In addition, the metal concentrations in the process baths (e.g. 130 g/L Cr6+ to 13 g/L Cr3+) lead to a calculated 90 % less hazardous waste (electroplating sludge). However, polyvalent carbon and boric acids in the waste water must be taken into account. Hauser + Walz GmbH from Flaach, Switzerland, is currently carrying out tests on the treatment of waste water with various chromium (III) electrolytes on behalf of the Federal Office for the Environment in Bern.
Summary
According to many companies that have converted bright chromium electrolytes to trivalent chromium, the advantages of the new process outweigh the disadvantages. For smaller companies with low throughput in chrome plating, however, the investment and analysis costs will be difficult to manage. The long time required for implementation in the company should not be underestimated. The legislation leaves it open as to how the substitution takes place; no wet-chemical process is prescribed. Therefore, the motto for the electroplating industry is "De Schnäller isch de Gschwindere" (...the faster is the slower). The substitution of hexavalent chromium is a rocky but rewarding road to greater progress in the company and greater acceptance by employees, the authorities and the public.
www.prowatech.ch
www.hauserwalz.ch


