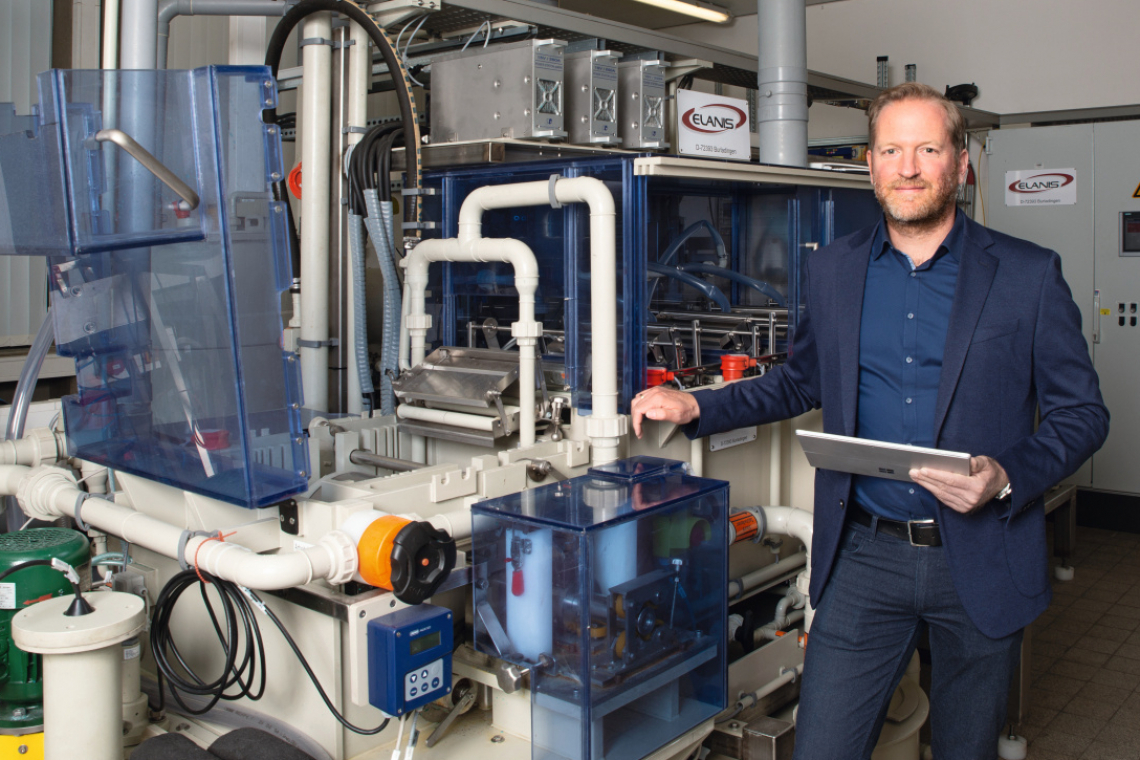
The composite electroforming process for battery cathodes developed at Aalen University successfully combines dispersion deposition with electroforming, thereby achieving significant advantages. Originally intended for lithium-sulphur batteries, the manufacturing process is now being further developed to meet the needs of industry. A team led by Prof. Dr. Timo Sörgel, Head of the Center for Electrochemical Surface Technology (ZEO) and Dean of Studies "Materials for Sustainability", is thus joining the race for the battery of the future.
In the age of electrification, battery technology is a key technology. It is the central anchor of the mobility and energy transition, which should lead to a climate-neutral economy and society by the middle of the century. Batteries power cars and mobile devices. Large battery storage systems will also store energy from wind and sun electrochemically for households, business and industry and provide it locally, while green hydrogen will replace gas and other fossil fuels for industry, for which immense capacities will have to be built up and extensive adjustments made to the infrastructure. Meanwhile, scientists in Germany and around the world are working on practicable solutions to adapt the technologies to the required demand. Development is in a state of flux: more advantageous developments can displace established solutions virtually from one day to the next.
An elegant new production method
The Center for Electrochemical Surface Technology (ZEO) at Aalen University is also taking part in this race for the energy technology of the future. The main protagonist is ZEO Director Prof. Dr. Timo Sörgel. In addition to the ZEO, he also heads the Surface and Materials Technology degree program at Aalen University and recently became Vice Dean of the Faculty of Mechanical Engineering and Materials Technology at Germany's most research-intensive university of applied sciences. Prof. Sörgel's team currently comprises five members of staff.
A conventional lithium-ion battery obtains its power storage function through a lacquer-like slurry coating made of a binder-conductive carbon black mixture for fixing and electrically contacting the active material made of mixed lithium nickel cobalt manganese oxides, among other things, which is applied to a metal foil that serves as a current collector. This creates the so-called cathode foil, which is then dried, compressed and folded or wrapped in the sequence cathode, separator, anode and finally forms the actual battery cell in a special housing, e.g. a so-called pouch cell, with the liquid electrolyte.
As early as 2012, work began in Aalen on the development of a "more elegant" production method, as Prof. Sörgel calls it: the composite electroforming of battery cathodes, for which the first prototype of a production plant was built together with the company Elanis. In this process, a metal matrix made of nickel is deposited on a temporary, rotating titanium cylinder substrate roller, into which sulphur particles are incorporated as active material by dispersion deposition. In the lower area of the layer, the metal also independently forms the particle-free so-called riding layer, which takes over the function of the current collector. The composite electroformed foil is continuously removed from the temporary titanium roller, leaving a self-supporting electroformed cathode foil. The elegant thing about the process is, on the one hand, the combination of galvanic dispersion deposition and electroforming and, on the other hand, that no binders or conductive additives are required as with the conventional battery cathode. There are only two components: the active material and the metal, which both binds mechanically and makes electrical contact. This enables higher power densities compared to the state of the art, which has a positive effect on the required charging times, for example. Composite electroforming is an environmentally friendly and both material- and energy-efficient process that is conceivable for all active materials, although this still needs to be developed and adapted for individual cases. Lithium-sulphur cells are generally considered to be comparatively safe, especially in terms of thermal runaway of the cell in the event of damage. In addition, this cell chemistry, which has not yet been commercialized, promises higher mass-related energy densities, which is particularly interesting for aerospace applications. In contrast, the lithium-sulphur battery cannot score points in electric cars because weight is currently of little importance there and the active materials of lithium-ion technology generally allow higher charging and discharging rates.
Lack of commercialization prevented the breakthrough
"Sulphur is abundant in the earth's crust, it is cheap and environmentally friendly and is even a waste product of other processes in large proportions. However, it has still not been commercialized in battery technology," says Prof. Sörgel, explaining the reason why a breakthrough in the battery market has not yet been achieved. This is actually surprising, as the storage capacity of the element is quite impressive with two electrons per sulphur atom. Other active materials only offer a quarter of this storage capacity. But where there is light, there is also shadow. "Sulphur is an insulator, so a lot has to be done to improve its conductivity," says Prof. Sörgel. This means that both the electrical contacting of the sulphur and the electrolyte in the lithium-sulphur battery play an important role, which in turn leads to disadvantages elsewhere - in this case in terms of fast-charging capability.
As promising as continued research into the lithium-sulphur battery appears, the research lead of over 30 years in lithium-ion batteries is difficult to catch up with. In discussions with the automotive industry, the composite electroforming process was certainly convincing, but not sulphur as the active material used. However, there is interest in using the process in the context of lithium-ion technology, as it also works with conventional active materials. However, a further reduction in weight is desired by using aluminum instead of nickel for the metal matrix. Composite electroforming is therefore currently being further developed with a different research focus. A specialist article by Philip Scherzl in the next issue of Galvanotechnik will show how far the project has progressed. "We still have to continue working on functionalization. We will have made a breakthrough if the electrodes behave as we postulate here. This still has to be shown in practice," comments Prof. Sörgel on the state of development of composite electroformed lithium-ion electrodes.
Shaping the electromobile future with electroplating technology
Oliver Kesten explains the exact functionality of the production system. He is a research assistant in Sörgel's team at the Center for Electrochemical Surface Technology and demonstrates the equipment in the electroplating training rooms. A transparent housing on the right-hand side of the construction separates the subsequent processes from the coating and rinsing area. Before completing his studies in surface and materials technology, Kesten learned the trade of electroplating, became a master craftsman and worked for years in the German Armed Forces in aircraft repair, including with hard chrome, electroless nickel and pre-treatments. He points to a wide roller over which the composite electroformed cathode foil is guided before it enters the subsequent processes. The electrolyte storage tank contains the nickel sulphamate-based nickel electrolyte required for dispersion deposition with functionalized sulphur particles dispersed in it. Before the composite galvanoformed film enters the downstream processes, a waterfall removes any loose particles. In the subsequent processes up to infrared drying, the rinsing stages are also separated by so-called air knives using compressed air in order to counteract carryover between the individual stages of the cascade rinse. A complicated but reasonably manageable process in a system that is only around 5 meters long and 1.5 meters wide.
Kesten is an electric car driver and is convinced that e-mobility will prevail and ultimately beat fuel cell technology and e-fuels. "Everything will depend on the costs," he says, referring to the increasing number of e-trucks at charging stations as well as ships and airplanes offering the first services with battery-electric drives. Today, the young expert is helping to shape the electromobile future. But what about the industrialization of the technology once the research into composite galvanoformed lithium-ion electrodes has been successfully completed?
Production technology is definitely competitive
"We once calculated what this could look like in a gigafactory and came to the conclusion that we are definitely competitive," reports Kesten. If conventional systems are compared with those for composite electroforming, conventional systems are significantly larger and coat faster than is possible with electroplating. However, conventional systems have very long drying sections, which are not necessary with the Aalen design. The systems from Aalen could simply be placed next to each other and produce in a highly automated manner. "The cost driver for us, as for others, is the use of materials," emphasizes Kesten. And this is likely to be lower without the binder-carbon black mixture that was previously used in conventional processes. The machine and plant costs, on the other hand, are hardly significant. In the TRL (Technical Readiness Level) classification, the system is already at level 6 out of 10.
 Cascade rinsing removes electrolyte and dispersoid residues (Photo: HS Aalen)
Cascade rinsing removes electrolyte and dispersoid residues (Photo: HS Aalen)
The so-called solid-state battery is also in the race for the battery of the future. Volkswagen recently announced a breakthrough here, and in Switzerland there are efforts to build a gigafactory for solid-state batteries with the Swiss Clean Battery. "Every approach has its justification," admits Prof. Sörgel, but also sees massive difficulties in development. "For a solid-state battery, a solid electrolyte would have to be used in order to be able to use elemental lithium. This is associated with a higher mass and impaired conductivity at low temperatures," he points out. According to him, the biggest problem in the development of such a battery is the phase boundaries from solid to solid, as the lithium ions move back and forth between the cathode and anode side during operation, which is associated with local increases and decreases in volume. "Then the phase boundaries delaminate and the contact is lost," he comments and states: "There will also be breakthroughs here, but with every new idea, as in our case, you always face new challenges."
 Infrared dryer for final drying of the film. Conventional systems require significantly longer drying distances than the system for composite electroforming (Photo: HS Aalen)
Infrared dryer for final drying of the film. Conventional systems require significantly longer drying distances than the system for composite electroforming (Photo: HS Aalen)
Prof. Sörgel sticks to what he does best: electrochemistry and electroplating dispersion deposition, which is also his main focus in Aalen. It is an area in which there are also extensive similarities with the Research Institute for Precious Metals and Metal Chemistry (fem) in Schwäbisch Gmünd. There was also cooperation on the lithium-sulphur battery, among other things. "We would now like to use our process for lithium-ion batteries, then we can fully exploit the potential, because lithium ions are designed for high C rates (a measure of the charging and discharging rates of a battery) and with our metallic matrix we can put the icing on the cake," the scientist is convinced.
How the spark is ignited
 In so-called glove boxes like these, the folded electrode foils are placed in a housing, the pouch cell. A vacuum is then created inside before the electrolyte is filled in and the cell is sealed (Photo: Robert Piterek)In general, Prof. Sörgel can certainly be seen as a "man of conviction" when you consider his career and commitment to date. Born in Kempten, he studied chemistry in Ulm and initially went into basic research at the Max Planck Institute for Solid State Research in Stuttgart after completing his doctorate. He was convinced by the entrepreneur Jürgen Meyer, who was also his superior for a while and is now Head of Business Development at De Martin AG. Meyer lit the spark. "Everything we touch is a surface," says Prof. Sörgel today. The position as a professor at Aalen University and working with students and his creative team at ZEO is his dream job. However, he is concerned about the declining number of students, even in traditional mechanical engineering. The young talent that the university trains is needed in industry, and only a few remain at the university. To increase student numbers, Prof. Sörgel has therefore sharpened the profile of his course. He is focusing on the potential of materials science and surface technology to find sustainable solutions for the future. But he doesn't want to stop there. Since this year, Prof. Sörgel has been a member of the board of the German Society for Electroplating and Surface Technology (DGO). "I want to see how we can make progress for the entire industry when it comes to young talent," he says and emphasizes: "Not everyone has to go to university, traditional vocational training is just as important, especially in Germany." He then says a few thought-provoking sentences that anyone in the industry would surely agree with. "I want young people to know that surface technology exists and what fascinating opportunities it offers. It would be a shame not to attract someone who was interested just because they didn't know about it."
In so-called glove boxes like these, the folded electrode foils are placed in a housing, the pouch cell. A vacuum is then created inside before the electrolyte is filled in and the cell is sealed (Photo: Robert Piterek)In general, Prof. Sörgel can certainly be seen as a "man of conviction" when you consider his career and commitment to date. Born in Kempten, he studied chemistry in Ulm and initially went into basic research at the Max Planck Institute for Solid State Research in Stuttgart after completing his doctorate. He was convinced by the entrepreneur Jürgen Meyer, who was also his superior for a while and is now Head of Business Development at De Martin AG. Meyer lit the spark. "Everything we touch is a surface," says Prof. Sörgel today. The position as a professor at Aalen University and working with students and his creative team at ZEO is his dream job. However, he is concerned about the declining number of students, even in traditional mechanical engineering. The young talent that the university trains is needed in industry, and only a few remain at the university. To increase student numbers, Prof. Sörgel has therefore sharpened the profile of his course. He is focusing on the potential of materials science and surface technology to find sustainable solutions for the future. But he doesn't want to stop there. Since this year, Prof. Sörgel has been a member of the board of the German Society for Electroplating and Surface Technology (DGO). "I want to see how we can make progress for the entire industry when it comes to young talent," he says and emphasizes: "Not everyone has to go to university, traditional vocational training is just as important, especially in Germany." He then says a few thought-provoking sentences that anyone in the industry would surely agree with. "I want young people to know that surface technology exists and what fascinating opportunities it offers. It would be a shame not to attract someone who was interested just because they didn't know about it."
Research at the cutting edge of development
The creativity of Prof. Sörgel and his team can also be seen in the last question on recycling, as the scientists from Aalen have also considered the recycling of the used material. Currently, batteries are often thermally decomposed and then concentrated and reused in many steps. ZEO, on the other hand, is working on reusing used electrodes as soluble anodes. "This is potentially much more elegant because we can get the material back into the process without using any additional energy," he argues.
There it is again, the word "elegant". In the context of electroplating, it has a good ring to it and makes it clear that the established surface process offers practicable - indeed elegant - solutions. Solutions that young scientists here - at the cutting edge of development - can help to devise and thus contribute to shaping the future.