Hard aluminum coating
The electrolytic plating of aluminum is not comparable to the plating of metals such as copper, nickel or zinc, especially when it comes to the bath composition. The plating of aluminum in molten salts and, more recently, in ionic liquids, is well known.
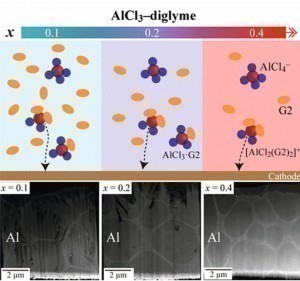 Fig. 1: Aluminum coating with a concentrated electrolyte (aluminum trichloride and diglyme)Scientists at Kyoto University in Japan, in collaboration with colleagues from Nanjing University in China, have developed a concentrated electrolyte consisting of aluminum trichloride and diglyme (diglycol dimethyl ether, G2) and electrolytically coated aluminum. The properties of the aluminum layer were investigated at different molar ratios AlCl3:G2 between 0.1 and 0.67. At low molar ratios, the microstructure was flake-like and characterized by micropores. At the molar ratio x = 0.4, the optimum value was reached and the layer microstructure was pore-free. The Al particles were spherical. At this molar ratio, there are hardly any neutral complexes, which is why the conductivity of the bath is relatively high, despite the high viscosity and high concentration. The degree of purity was > 99.0% Al. The nanohardness of the layer was 2.86 GPa and was relatively three times higher than that of Al layers coated from a typical Al bath with ionic liquids. Thanks to the <100> crystal orientation, the coating was non-porous and had very good corrosion resistance [1].
Fig. 1: Aluminum coating with a concentrated electrolyte (aluminum trichloride and diglyme)Scientists at Kyoto University in Japan, in collaboration with colleagues from Nanjing University in China, have developed a concentrated electrolyte consisting of aluminum trichloride and diglyme (diglycol dimethyl ether, G2) and electrolytically coated aluminum. The properties of the aluminum layer were investigated at different molar ratios AlCl3:G2 between 0.1 and 0.67. At low molar ratios, the microstructure was flake-like and characterized by micropores. At the molar ratio x = 0.4, the optimum value was reached and the layer microstructure was pore-free. The Al particles were spherical. At this molar ratio, there are hardly any neutral complexes, which is why the conductivity of the bath is relatively high, despite the high viscosity and high concentration. The degree of purity was > 99.0% Al. The nanohardness of the layer was 2.86 GPa and was relatively three times higher than that of Al layers coated from a typical Al bath with ionic liquids. Thanks to the <100> crystal orientation, the coating was non-porous and had very good corrosion resistance [1].
Robust anti-fog nanocoating
A unique property of pin or pad electroplating is the fact that any number of nanolayers of the metal can be coated. This changes the coating properties in a positive sense.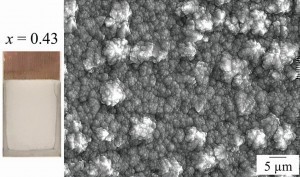 Fig. 2: (a) Appearance of the Al coating and (b) SEM image of the Al coating (at -1.0V and x = 0.43)
Fig. 2: (a) Appearance of the Al coating and (b) SEM image of the Al coating (at -1.0V and x = 0.43)
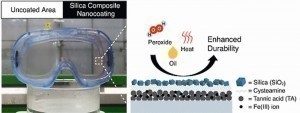
Scientists at Ewha Womens University in Seoul, South Korea, inspired by biomimetic examples, have done something similar. Silica composite nanofilms were applied in several layers to a base layer of the versatile Fe(III) tannic acid nanofilm.
The thickness of the nanofilm layer could be precisely controlled and had an influence on the anti-fogging properties. Due to the superhydrophilic properties of the SiO2 layer, the anti-fogging effect was very clearly visible.
The developed coating combination was resistant to the effects of acids, heat, heat and oil contamination. The nanocoating was generated by means of a spray process [2].
 Fig. 3: SEM images of the galvanostatic coating of Al at x = 0.1, 0.2 or 0.4 and 15 mA.cm-2
Fig. 3: SEM images of the galvanostatic coating of Al at x = 0.1, 0.2 or 0.4 and 15 mA.cm-2
 Fig. 5: Anti-fogging test: (a) exterior mirror and (b) safety goggles
Fig. 5: Anti-fogging test: (a) exterior mirror and (b) safety goggles
![Bild 6: Anti-Beschlag Performance-Test mit Glas ohne Beschichtung, mit einer [Fe(III)-TA]6- Beschichtung und mit einer [[Fe(III)- TA]2/Cys50/SiO2]3-Komposit-Beschichtung](/images/stories/Abo-2020-10/thumbnails/thumb_gt-2020-10-0028.jpg) Figure 6: Anti-fog performance test with glass without coating, with an [Fe(III)-TA]6 coating and with an [[Fe(III)- TA]2/Cys50/SiO2]3 composite coating
Figure 6: Anti-fog performance test with glass without coating, with an [Fe(III)-TA]6 coating and with an [[Fe(III)- TA]2/Cys50/SiO2]3 composite coating
Insignia cards in Covid-19 times
Insignia, a company specializing in lifestyle and luxury management, has developed a mechanical coating process to keep surfaces germ-free with the help of a silver layer.
The process is used in Insignia cards. Around 5 million credit cards are active in the Emirates. According to a study, around 11% of credit cards have bacteria on their surface. Titanium or zinc surfaces work best as a substrate.
According to Insignia, the application possibilities can be extended to cell phones, laptops, office supplies and more.
Address of the author
Dr. Nagaraj N. Rao
RRR House, RRR Labs Pvt. Ltd.
Plot 80, Sector 23,
Navi Mumbai - 400 705 India
E-mail:
Literature
[1] ACS Appl. Mater. Interfaces 2020, 12, 38, pp. 43289-43298[2] ACS Appl. Mater. Interfaces 2020, 12, 37, pp. 42109-42118