The galvanic deposition of dispersion layers has been known for around a hundred years. The particles to be deposited are added to a metal matrix electrolyte in the form of powders or as a suspension and kept in suspension by pumping, stirring, air injection, etc. By selecting suitable electrolyte additives and deposition parameters, particles can be incorporated into the coating [1].
 Silver dispersion deposition in practiceDiamondparticles were embedded in nickel coatingsas early asthe 1930s, e.g. for applications in dentistry or as a coating for nail files [2]. Over the years and decades, almost countless metal-dispersoid combinations have been deposited and characterized for a wide variety of applications. Based on the initially empirical experiments, various models were developed and discussed from the 1960s onwards to describe the mechanism of co-deposition [1, 3-5]. A widely known model was developed by Gugliemi in 1972, which is based on a two-stage adsorption mechanism and takes particle and electrolyte properties into account. Other authors have extended or refined this model to include other influencing parameters, such as current density [6] or hydrodynamic conditions [7]. There are a number of other approaches, e.g. that of Celis and coworkers, which allows the estimation of dispersoid incorporation at a certain current density. A more detailed description of the various models can be found in [1] and [5].
Silver dispersion deposition in practiceDiamondparticles were embedded in nickel coatingsas early asthe 1930s, e.g. for applications in dentistry or as a coating for nail files [2]. Over the years and decades, almost countless metal-dispersoid combinations have been deposited and characterized for a wide variety of applications. Based on the initially empirical experiments, various models were developed and discussed from the 1960s onwards to describe the mechanism of co-deposition [1, 3-5]. A widely known model was developed by Gugliemi in 1972, which is based on a two-stage adsorption mechanism and takes particle and electrolyte properties into account. Other authors have extended or refined this model to include other influencing parameters, such as current density [6] or hydrodynamic conditions [7]. There are a number of other approaches, e.g. that of Celis and coworkers, which allows the estimation of dispersoid incorporation at a certain current density. A more detailed description of the various models can be found in [1] and [5].
It is undisputed that a large number of influencing variables have an effect on the co-deposition of particles, e.g. deposition conditions, hydrodynamics, electrolyte composition and additives, type and concentration of particles [6].
Conductive dispersion layers
For applications in the field of electrical contacts, silver is a suitable matrix for dispersion layers. Silver has the highest electrical conductivity of all the elements, together with good corrosion resistance and a comparatively low price for precious metals. The disadvantages of silver are its tendency to weld, its low tarnish resistance and its low hardness [8]. Alloys or composite materials are a good alternative to the use of pure silver, especially for reducing the tendency to cold welding. Systems known from powder metallurgy are silver-nickel and silver-graphite. In the silver-graphite system, the tendency to weld is greatly reduced by the incorporation of graphite particles. At the same time, graphite has a very good lubricating effect, which is why the tribological properties are also significantly improved when incorporated parallel to the surface. According to [9], the silver-graphite system in the form of a galvanically deposited dispersion layer was first mentioned in the patent specification DE-AS1496927, where it is not used as a functional layer, but as a structuring underlayer to produce micro-cracked chrome layers.
Siemens takes on a pioneering role
 Silver-graphite coated component before (left) and (right) after ball burnishingTheactual development of electroplated silver-graphite dispersion layers for use in electrical contacts is closely linked to Siemens. The first published patent for this was registered in 1975 [9]. It describes a cyanide silver electrolyte in which graphite particles of 1-10 µm are dispersed. The particles are kept in suspension by pumping the electrolyte around. Depending on the graphite concentration in the electrolyte, the dispersion layers deposited in this way can contain up to 3 percent graphite by weight and exhibit excellent abrasion resistance, making them suitable for use in relay contacts.
Silver-graphite coated component before (left) and (right) after ball burnishingTheactual development of electroplated silver-graphite dispersion layers for use in electrical contacts is closely linked to Siemens. The first published patent for this was registered in 1975 [9]. It describes a cyanide silver electrolyte in which graphite particles of 1-10 µm are dispersed. The particles are kept in suspension by pumping the electrolyte around. Depending on the graphite concentration in the electrolyte, the dispersion layers deposited in this way can contain up to 3 percent graphite by weight and exhibit excellent abrasion resistance, making them suitable for use in relay contacts.
Another Siemens patent from 1991 [10] describes a process for the deposition of silver-graphite layers in which higher current densities can be used. In contrast to the earlier patent [9], the electrolyte no longer contains free cyanide, but alternative conductive salts such as potassium hydrogen phosphate, potassium phosphate or sodium citrate. With this formulation, current densities of up to 20 A/dm2 can be achieved and the coatings can be applied in a continuous system. As explained in [11], the silver-graphite surface at Siemens has developed over the years or decades into a commercial product with a wide range of applications. Only in 2021, due to the closure of the in-house technology center, its production was discontinued or transferred to Pieper Oberflächentechnik GmbH Hermsdorf.
Parallel to silver graphite, Siemens also transferred coatings made of silver nanodiamond to industrial scale to increase wear resistance [11]. Patents [12] and [13] describe both a process for the corresponding preparation of nanodiamonds and the deposition of a corresponding dispersion layer from a cyanide electrolyte.
According to [14], potassium hexacyanoferrate-based electrolytes can also be used for the co-deposition of nanodiamonds.
Literature mentions other dispersoids
In addition to graphite and nanodiamond, numerous other dispersoids that have been incorporated into silver matrices are described in the literature and in patents. These include, for example, other carbon-based materials such as graphene or carbon nanotubes [15] as well as PTFE [17], zirconium oxide [16] and many more. However, these apparently did not achieve the same industrial relevance as silver graphite.
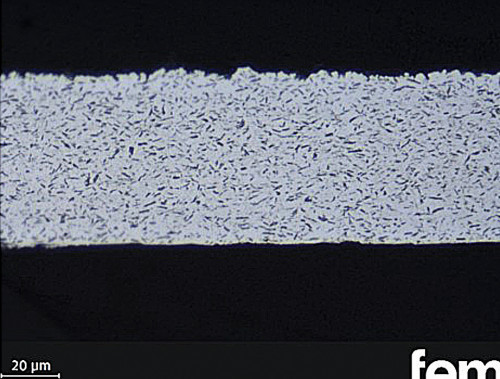
Due to the constantly increasing demands on contact surfaces and the opening up of new fields of application, for example in the field of e-mobility, silver dispersion layers continue to be the subject of research work. For example, graphite particles have been successfully incorporated into silver layers made from cyanide-free electrolytes [18]. Furthermore, the substitution of graphite with alternative self-lubricating particles and the resulting properties of these coatings are currently being investigated as part of a joint project of the Federal Ministry of Economics and Climate Protection (BMWK) [19]. The cross-sectional images of silver dispersion layers in this article show Ag graphite on the left and Ag-hBN (hexagonal boron nitride) on the right. This means that even fifty years after their "discovery", silver dispersion layers have lost none of their attractiveness and topicality and represent a useful alternative for current issues.
Literature
[1] Meyer, J.; Sörgel, T.: Chemical and electrochemical dispersion coating, WOTech, Vol. 2013, No. 9, https://www.wotech-technical-media.de/womag/ausgabe/2013/09/24_w_soergel_dispersion_09j2013/24_w_
soergel_dispersion_09j2013.php
[2] Praktische Galvanotechnik, 1988, 4th edition, Eugen G. Leuze-Verlag, p. 279
[3] Wielage, B.; Lampke, T.; Dietrich, D.; Zacher, M.: Abscheidung und Werkstoffaufbau galvanischer Dispersionsschichten; Werkstoffe und werkstofftechnische Anwendungen Volume 31, Ed.: B. Wielage, 11th Materials Technology Colloquium, Chemnitz 2008, pp. 135-140
[4] Walsh, F.C. et al.: A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: an established and diversifying technology, Transactions of the IMF 92 (2014) 2, pp. 83-98
[5] Low, C.T.J.; Wills, R.G.A.; Walsh, F.C.: Electrodeposition of composite coatings containing nanoparticles in a metal deposit, Surface & Coatings Technology 201 (2006), pp. 371-383
[6] Hwang, B.J.; Hwang, C.S.: J. Electrochem. Soc. 140 (1993) 979, cited in [5]
[7] Berçot, P.; Pena-Munoz, E.; Pagetti, J.: Surf. Coat. Technol. 157 (2002) 282, , cited in [5]
[8] Keil, A.: Electrical contacts and their materials; Springer-Verlag, Berlin, 1984
[9] DE2543082: Cyanide silver electrolyte for the galvanic deposition of silver-graphite dispersion coatings
[10] DE 4010346: Process for the application of silver-graphite dispersion coatings
[11] Dambrowsky, N.: Silver graphite - more than just silver deposition, Galvanotechnik 5 (2021), pp. 589-593
[12] DE10125290: Process for the preparation of nanodispersants
[13] DE10125289: Process for producing an abrasion-resistant, electroplated layer on parts and parts produced thereafter
[14] Kuzjmar, I. et al.: Galvanic dispersion coating of electronic components based on silver; PLUS 5 (2008), pp. 1039-1045
[15] Litovka, Y. et al.: Electrodeposition of MWCNTs / silver composite coatings with enhanced mechanical characteristic, IOP Conference Series: Materials Science Engineering, 693 (2019) 012005
[16] Gay, P.-A.; Berçot,P.; Pagetti, J.: Electrodeposition and characterization of Ag-ZrO2 electroplated coatings; Surface and Coatings Technology 140 (2001), pp. 147-154
[17] US2016032479: Electrodeposition of silver with fluoropolymer nanoparticles
[18] https://www.zvo.org/aktuelles/detailansicht/herstellung-und-charakterisierung-galvanischer-silber-graphitdispersionsschichten-aus-einem-cyanidfreien-elektrolyten, ZVO report 3/2021, Master thesis Jan Thiergarten, TU Ilmenau, 2021, retrieved on 06.05.2022
[19] BMWi 03EI6011C: Contact and long-term behavior of self-lubricating coatings in current-carrying connections in electrical power engineering (SEBEEL), duration 01.09.2019-31.08.2022


![Querschliffaufnahme von Silberdispersionsschichten; links: Ag-Graphit-Schicht, rechts: Ag-hBN (hexagonales Bornitrid) [19] Querschliffaufnahme von Silberdispersionsschichten; links: Ag-Graphit-Schicht, rechts: Ag-hBN (hexagonales Bornitrid) [19]](/images/stories/Abo-2022-08/thumbnails/thumbnails/thumbnails/thumb_gt-2022-08-0100.jpg)