High-performance thermoplastics such as polyether ether ketones (PEEK) have similar mechanical properties to human bone. This makes PEEK a promising plastic for medical bone replacement applications. In order to increase the bioactivity of PEEK, hydroxyapatite (HAp) layers can be deposited on PEEK surfaces using cold plasma spraying. The deposited and adhesive HAp layers have a porous structure that is suitable for the growth of bone cells.
Introduction
PEEK is a promising biomaterial for postoperative visualization due to its low modulus of elasticity and its permeability to X-rays. Compared to metallic biomaterials such as stainless steels and titanium alloys, PEEK has similar mechanical properties to human bone to avoid the stress shielding effect at the bone/implant interface [1]. However, its bioinert surface can lead to fibrous encapsulation and poor osseointegration.
In order to increase the bioactivity of PEEK, modifications of the material surfaces are often necessary. Torstrick et al. increased the bioactivity of the PEEK material by creating a porous surface of the PEEK [2]. Hahn et al. applied a hydroxyapatite layer to PEEK intervertebral implants by aerosol deposition to create an adherent bioactive surface [3]. It is also possible that a HAp layer can be deposited on PEEK substrate by conventional atmospheric spraying [4, 5].
However, a compromise must be found between overheating of the substrate and layer formation [6]. In order to minimize the thermal load on the substrate, the hydroxyapatite layers can be deposited on thermally sensitive substrates by means of atmospheric cold plasma spraying. Cold plasma spraying is a modified form of plasma spraying in which very fine powders are melted at moderate power and accelerated onto surfaces to be coated.
Material and methods
Platelets made of PEEK in the dimensions Ø 17 mm x 2 mm were used as substrate materials (Rocholl GmbH, Eschelbronn, Germany). The melting temperature of this plastic is 341 °C. Before coating, all samples were first sandblasted with corundum to achieve a surface roughness Sq of 4 µm. All samples were then cleaned with isopropanol in an ultrasonic bath for 15 minutes and then dried at room temperature. The spray material used was hydroxyapatite (HAp) powder with a particle size D50 of 27 µm (MediCoat AG, Étupes, France). The purity of the HAp phase was > 95 %. As a spray additive for medical applications, this powder meets the requirements of ASTM f1185 and ISO 13779. The powder contains a minimal amount of calcium oxide (less than 1 %).
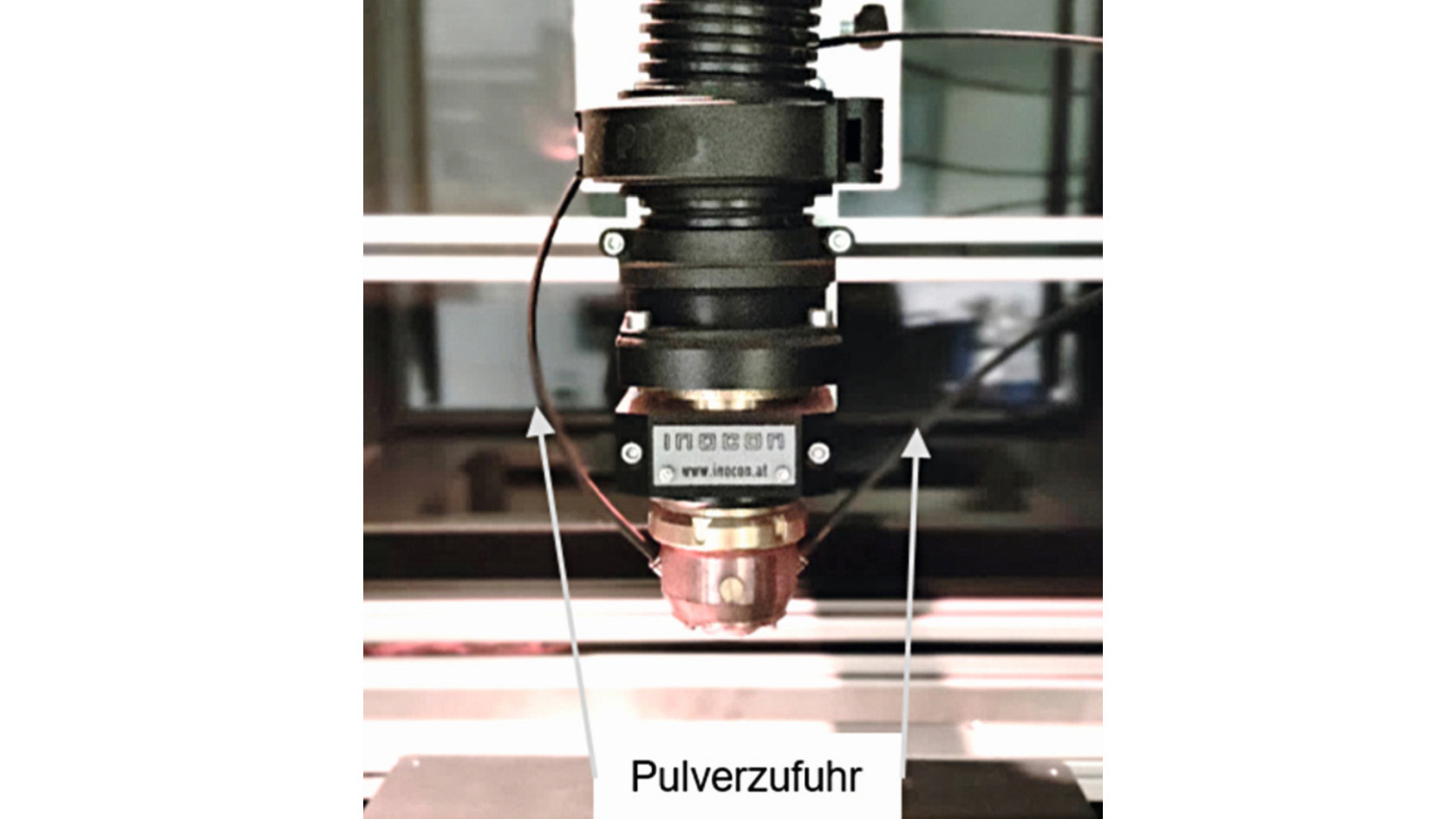
The coating and the prior activation were carried out using the cold plasma system IC-3 (Inocon, Attnang-Puchheim, Austria). A diagram of the cold plasma system is shown in Figure 1. In the device, an electrical discharge takes place between a centrally arranged tungsten cathode and the nozzle serving as the anode. The adjustable power range extends from 1 to 11 kW. For the tests presented here, a power of 5.7 kW (250 A, 23 V) was used in order to avoid damaging the PEEK substrate. During the coatings, the PEEK surface was scanned with the plasma in a meandering pattern, whereby the substrate was moved at a speed of 250 mm/s relative to the outlet opening of the plasma nozzle using an x-y traversing table. Table 1 provides an overview of the parameter set used.
|
Process gas |
Argon @ 10 L min-1 |
|
Carrier gas |
Argon @ 7 L min-1 |
|
Amperage |
250 A |
|
Voltage |
23 V (automatically regulated) |
|
Spray distance |
44 mm |
|
Relative movement of the substrate |
250 mm s-1 |
|
Powder feed rate |
3 g min-1 |
|
Grid/offset |
4 mm / 2 mm |
|
Number of passes |
10 |
A pronounced increase in temperature was to be expected when coating the PEEK substrate. The process control in correlation to the surface temperature achieved was carried out accordingly by means of temperature measurement during plasma spraying. The temperature measurement during activation and the subsequent coating was carried out using a K-type thermocouple, which was placed 1 mm below the PEEK surface in the hole. Pause times were observed between two plasma treatment runs in order to achieve a moderate surface temperature.
The bond strength of the HAp layer was tested by means of a compression shear test using the testing machine from Hegewald & Peschke (Nossen, Germany). Before the test, the sandblasted stamp (Ø 5 mm) was flamed with Pyrosil and then coated with the primer GP15 (Sura Instruments, Jena, Germany). The stamps and the samples to be tested were then bonded using Scotch-Weld DP460 (3M, St. Paul, USA) and cured at 65 °C for 3 hours (fivefold determination).
The morphological examinations of the HAp layers produced were carried out using scanning electron microscopy. The phase composition was determined using X-ray diffraction (XRD).
Results and discussion
The primary objective, in addition to the adhesion-resistant deposition of the coatings on PEEK, was to avoid high thermal loads. This was made possible by selecting suitable process parameters. During activation and coating, the temperature-time profile was recorded with a K-type thermocouple (Ø 0.25 mm, TC Direct, Mönchengladbach, Germany) (Fig. 2). This figure shows the temperature profile 1 mm below the surface at a plasma power of 5.7 kW. Note the areas circled in red, which highlight the maximum temperature of the respective pass (DL). Due to the extremely short exposure time below the plasma jet, neither optical nor physical damage to the substrate was caused. In order to evaluate the thermal load on the material, the maximum temperatures reached in each run were considered. Figure 2 shows the temperature-time profile at the plasma power of 5.7 kW, the spray distance of 44 mm and the substrate speed of 250 mm/s. Temperatures of up to 69 °C were reached after activation. Pause times of 6 minutes were observed between activation and 2 runs. The maximum temperature of up to 110 °C was reached after 10 passes. The application temperature of PEEK is permissible at up to 260 °C [7]. The temperature profile achieved should therefore not be regarded as critical.
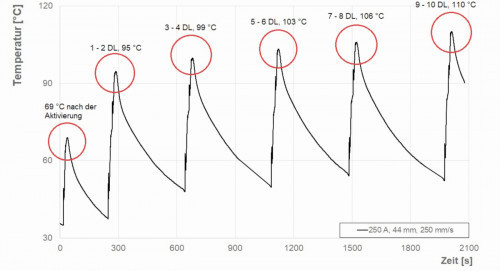 Fig. 2: Temperature as a function of time during activation (1 DL) and coating (10 DL); the maximum temperatures of the respective run (DL) are highlighted in red circles
Fig. 2: Temperature as a function of time during activation (1 DL) and coating (10 DL); the maximum temperatures of the respective run (DL) are highlighted in red circles
SEM examinations of the HAp powders reveal a slightly irregular shape (Fig. 3 left). Due to the relatively low plasma power (5.7 kW), the HAp powders could not be completely melted. Figure 3 (right) shows that the core of the HAp powders, which were heated in the plasma, had an unchanged structure. It could thus be demonstrated that only the surface of the HAp powders is melted.
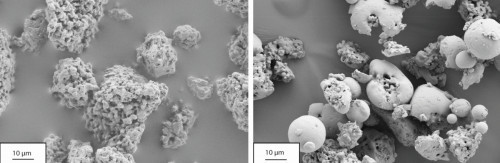 Fig. 3: Morphology of the HAp powder in its original state (left) and through the plasma (right)
Fig. 3: Morphology of the HAp powder in its original state (left) and through the plasma (right)
The deposited HAp layers had a rough surface, which could completely cover the underlying substrate (Fig. 4 left). Microcracks were located at the smooth areas where the hydroxyapatite was melted. Crystallites on the surface indicate non-melted components (core of the HAp particles). These not completely melted particles led to a porous structure of the HAp layer (Fig. 4 right). The results of X-ray diffraction confirmed that the crystallinity of the sprayed HAp layer was 83 % (initial HAp powder 95 %). Hydroxyapatite as the main component (JCPDS 9.432) was shown in the spectrum (Fig. 5). No peaks of α-TCP, β-TCP or tetracalcium phosphate (TTCP) (JCPDS 9.348, 9.169, and 25.1137, respectively) were detectable. Minimal components of magnesium oxide and calcium oxide were found in the spectra, as magnesium and calcium oxide were present as impurities in the spray additive. (Fig. 4 left).
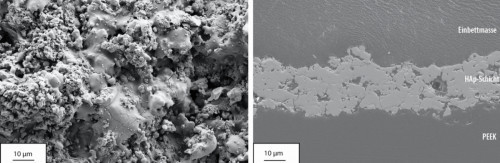 Fig. 4: Top view image (left) and cross-section image (right) of the HAp layer on PEEK (at 5.7 kW, spray distance of 44 mm, scanning speed 250 mm/s)
Fig. 4: Top view image (left) and cross-section image (right) of the HAp layer on PEEK (at 5.7 kW, spray distance of 44 mm, scanning speed 250 mm/s)
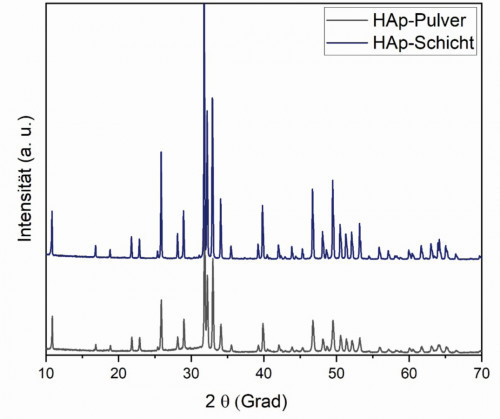 Fig. 5: XRD spectra of HAp powder and coating
Fig. 5: XRD spectra of HAp powder and coating
Cross-sectional micrographs (Fig. 4 right) showed good bonding at the interface between the hydroxyapatite and the PEEK substrate. The HAp layer achieved on PEEK exhibits good overall adhesion. Its shear strength is 43.4 MPa. Defects on the PEEK substrate itself could not be observed (holes, embrittlement). The temperature-time profile could therefore be considered a valid method for process monitoring.
Conclusion and outlook
The high-performance plastic polyether ether ketone (PEEK) is an almost ideal material for applications in the field of implant technology. However, its bioinert surface is its biggest disadvantage. These surfaces can be modified with the help of bioactive hydroxyapatite. It has been shown that cold plasma spraying is suitable for depositing adhesive bioactive HAp layers on PEEK materials that are relevant for medical applications without causing optical or physical damage. Due to the low and easily adjustable plasma power, HAp particles were not completely melted in the plasma. This led to a porous layer structure with very high crystallinity, which is the ideal basis for high compatibility with bone cells.
In future studies, the biocompatibility and cytotoxic behaviour of the HAp layers will be characterized in more detail. In addition, further functionalities are to be added directly during the coating process. Among other things, the deposited HAp layers can be supplemented with zinc oxide, which can be simultaneously integrated into the HAp layers using atmospheric pressure plasma chemical vapor deposition (APPCVD). In this way, layers with optimal conditions for the body's own cells are to be created, which, however, have unfavorable growth conditions for exogenous microorganisms.
Acknowledgments
Part of the work presented here was supported by the German Federal Ministry for Economic Affairs and Energy (BMWi) under grant number 49MF200039.
Literature
[1] J.M. Toth et al.: Polyetheretherketone as a biomaterial for spinal applications, Biomaterials Volume 27, Issue 3 (2006) 324-334
[2] F.B. Torstrick et al: Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK, Biomaterials 185 (2018) 106-116
[3] Byung-Dong Hahn et al: Osteoconductive hydroxyapatite coated PEEK for spinal fusion surgery, Applied Surface Science 283 (2013) 6-11
[4] R.B. Heimann: Thermal spraying of biomaterials, Surf. Coat. Technol., Vol 201, N°5, 2006, 2012-2019
[5] Y.C. Tsui; C. Doyle; T.W. Clyne: Plasma sprayed hydroxyapatite coatings on titanium substrates, Part 1: Mechanical properties and residual stress levels, Biomat., Vol 19, 1998, 2015-2029
[6] P. Robotti et al: Thermal Plasma Spray Deposition of Titanium and Hydroxyapatite on PEEK Implants, PEEK Biomaterials Handbook, 2019
[7] S. Beauvais et al: Plasma Sprayed Biocompatible Coatings on PEEK Implants, Thermal Spray 2007: Global Coating Solutions