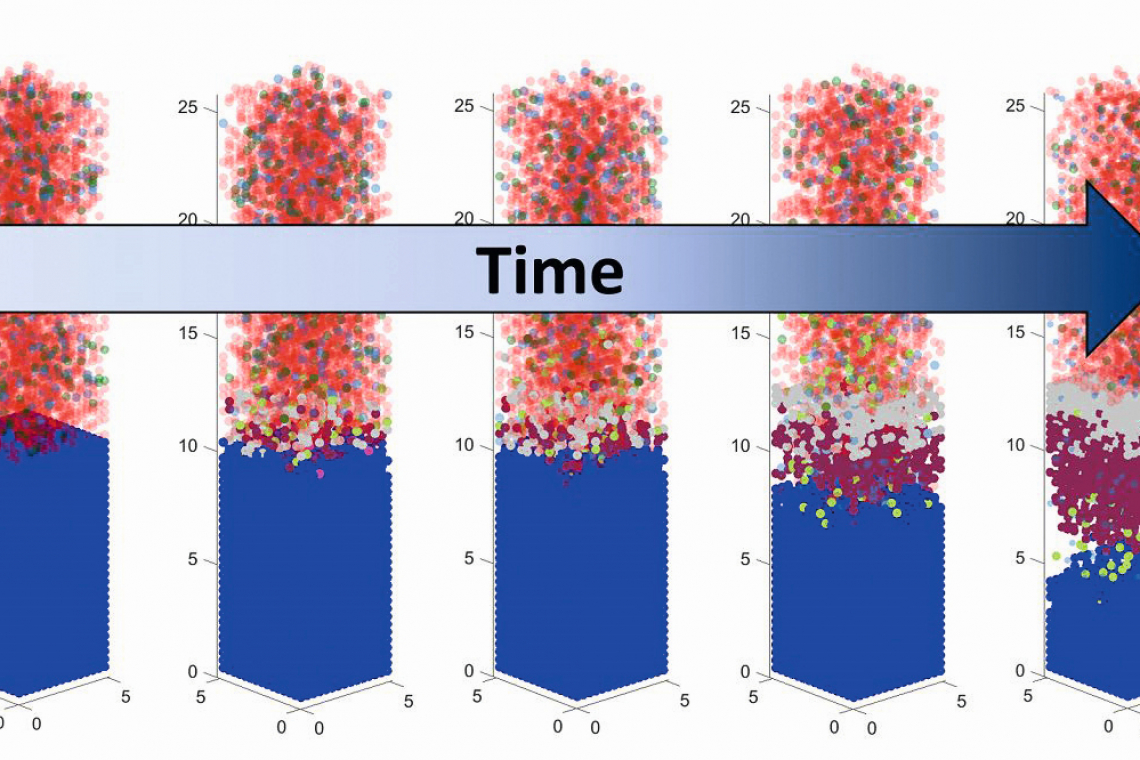
A promising candidate for future energy storage is the lithium metal battery. Compared to conventional lithium-ion batteries, it has several times the energy density and a higher specific capacity. One limitation in its application to date has been the reactivity of the lithium metal electrode with the liquid electrolyte. A solid passivation layer forms at the interface between the electrode and the electrolyte, which was previously difficult to control and stabilize.
"With the new simulation tool, it is possible to show in molecular resolution how the passivation layer forms on the lithium metal within the first microseconds after contact with the liquid electrolyte," explains Janika Wagner-Henke from the Institute for Applied Materials - Electrochemical Technologies at KIT. The tool can be used to understand molecular processes that were not accessible using conventional molecular dynamics simulation methods or experiments.
Understanding the complex interfacial processes enables the knowledge-based design of the passivation layer (solid electrolyte interphase, SEI for short). A stable SEI on lithium metal electrodes is an important prerequisite for making it usable on a large scale. The simulation tool presented creates an important basis for this and is a further step towards high-performance and long-lasting lithium metal batteries.