Explosion protection in companies with explosive solvents and dusts is usually formal at best. Personal experience with explosions usually remains in the dark, especially in the absence of major damage. But even the causes of major explosion accidents are all too often not made public, to the detriment of the many companies that have suffered similar disasters later on.
For insurance reasons and for fear of legal consequences and reputational damage, the causes of explosions usually remain hidden despite extensive efforts. Explosion protection is more than just complying with legal and sub-legal regulations: it also includes learning from incidents, sharing relevant experience and extensive training, especially in the area of preventive explosion protection.
4 Explosion protection measures
Protective measures are an important chapter in explosion protection. Safety experts distinguish between primary, secondary and tertiary explosion protection measures.
4.1 Primary protective measures
Fig. 14: Explosion area of the gas mixture methane/air/oxygen/inert gas (N2, argon)Primary explosion protection describes measures that prevent the formation of an explosive situation from the outset.
The avoidance of an explosive atmosphere, for example, must be brought about systematically. This can also be achieved by substituting solvents and explosive gases.
If it is not possible to replace the reactants with less explosive components, mixtures with inert gases such as nitrogen or argon, and sometimes also water vapor or carbon dioxide, can be useful. For example, the explosion limits of methane in air with an oxygen content of 21% by volume are reduced to 4.4% and 19% by volume (Fig. 14). From an inert gas content of 40% by volume, an explosion is no longer to be expected [16].
Reaction matrices for production areas are also a helpful primary protective measure: which substances can come together in intended and non-intended operation. This makes it easier to recognize physical explosions and incompatible systems and, if necessary, to check them by means of expert opinions or tests.
An important focus is also on preventive safe storage: storage concepts take into account wide distances or isolated storage of explosive substances as well as prohibitions on combined storage.
Fig 15: Warning sign for a hazardous industrial area (Photo: CEphoto, Uwe Aranas)Preliminary research into the explosive decomposition of chemicals under the influence of various parameters, such as temperature, pressure, atmosphere or highly exothermic reactions, such as polymerization, oxidation, neutralization, also helps to control processes under all circumstances.
Finally, an almost trivial safety measure should be mentioned. However, since it is sometimes given little consistent attention, it is all the more dangerous: strict access control for hazardous areas. How easy it is for colleagues from other areas, visitors, customers, etc. to enter the hazardous area quickly and unsupervised without observing all safety-related behavioral measures. It is therefore very important that the Ex areas are comprehensively marked and maintained (Fig. 15).
4.2 Secondary protective measures
Secondary explosion protection prevents an explosive atmosphere from exploding. This involves preventing ignition sources or rendering them ineffective.
To prevent an explosion in the designated Ex zones, no effective ignition sources may be used. Any possibility of ignition must be rigorously and permanently excluded. System operators must therefore constantly remind themselves of the potential ignition sources, ideally with the help of a checklist.
Special precautions must be taken during maintenance work. Permission slips (access or fire permits) must be filled out and discussed with particular care.
The higher and longer the probability of a hazardous explosive atmosphere occurring, the higher the requirements for the equipment used there. The extent of a potentially explosive zone depends on the quantity of the substance in question and the secondary explosion protection measures introduced, such as ventilation or gas warning systems.
Furthermore, the specific properties of the substance must be taken into account when defining the zone: These include maximum temperatures at the system parts, density in relation to air, lower and upper explosion limit, maximum explosion pressure and pressure build-up speed.
Fig. 16: Densities of gaseous operating fluids in relation to atomic/molecular weight To ensure that extraction systems are used in the right places, the densities of hazardous gases are important in relation to air density, taking into account the temperatures (Fig. 16): Hydrogen, methane and ammonia, for example, are significantly lighter than air at room temperature and can accumulate in less ventilated ceiling areas.
Ventilation measures can prevent explosion limits from being reached in the Ex zones, or an Ex zone with lower requirements is sufficient. It is also possible to switch on suitable room or object ventilation systems or switch off non-explosion-protected equipment via appropriate gas sensors if a limit value is exceeded. Companies at risk of explosion with explosive liquids or gases initiate such safety measures at 50% of the lower explosion limits in question.
Explosive solids can be subcooled, kept moist or heavily diluted with inert materials.
4.3 Tertiary protective measures
Explosion risks cannot always be completely avoided by selecting suitable primary and secondary protective measures. In this case, tertiary, i.e. additional explosion protection measures are inevitably used to control and limit the effects of an explosion. Material damage should remain within narrow, manageable limits, and any risk to employees must be completely ruled out.
Tertiary explosion protection measures must be taken, for example, when pneumatically conveying explosive dusts or in silos and bunkers with organic powders where the formation (friction, electrostatics) or introduction of glow nests (sparks) is possible.
Fig. 17: Rupture disk (bursting diaphragm) adapted to special overpressure after the explosion (A), in the original (B)The following protective measures are possible and must be checked for their special suitability [16]:
- constructive explosion protection through explosion pressure or explosion pressure shock resistant construction of apparatus and structures that can withstand the explosion pressure
- Installation of flame arresters (metal screens) that cool down a flame front in pipes to such an extent that an explosion remains spatially limited
-
Flame arresters in pipelines by immersing them in water, which interrupt an explosion flame front
-
Automatic quick-closing devices in conjunction with suitable IR detectors that close valves in connected pipelines with a sufficiently short reaction time
-
Self-contained shut-off valves in pipelines for gases, which shut off sections of the pipeline in the event of a sudden increase in pressure
-
Explosion suppression using cooling, inerting extinguishing agents that are sprayed suddenly into the system to be protected (e.g. silo) after activation
-
Pressure relief devices, such as pressure relief flaps or bursting disks (Fig. 17), which limit the explosion pressure to a manageable level. Relatively large venting areas are required, which can be blown off into the open without risk.
5 Appliances in potentially explosive atmospheres
Devices that can be used in potentially explosive atmospheres are divided into three groups in accordance with European standard EN 60079-0:2009. The device categories that can be used in these zones are based on the Ex zone determined.
5.1 Device groups
Group I stands for "mines at risk of firedamp", as found in coal mining. It depends on the explosive substance in question. The hazard increases from IA to IC.
Group II stands for "explosive gases" with subgroups IIA, IIB and IIC. Subgroup A includes, for example, diesel, gasoline, ethane, methane and carbon monoxide. Subgroup B includes, for example, town gas (methane) and ethylene.
Subgroup C includes hydrogen, acetylene and carbon disulphide.
Group III contains explosive dusts. This includes both organic powders and inorganic, easily oxidizable metal powders. The standard provides subgroups IIIA for fibers, IIIB for non-conductive dusts and IIIC for conductive dusts.
5.2 Labeling
Equipment categories are defined in the ATEX Directive 2014/34/EU. Internationally, the term "Equipment Protection Level", EPL, is used for the term "equipment protection level" [29].
According to the ATEX Directive 94/9/EC, the letter "G" stands for gas and "D" for dust.
Devices of category 1G/1D or EPL Ga/D may be used in zone 0 (category 1G) or zone 20 (category 1D). At this highest level of protection, even two faults on the appliance must not lead to ignition.
Devices of category 2G/2D or EPL Gb/Db are suitable for zone 1 (category 2G) or zone 21 (category 2D).
Devices of category 3G/3D or EPL Gc/Dc meet the requirements of Ex zones 2 (category 3G) or zone 22 (category 3D).
6 Summary
Explosions are the most horrific events that can occur in production facilities. Therefore, explosion protection is characterized by a multitude of laws, standards, characteristics and proposals.
Unfortunately, these already complex formal contexts and procedures are only one side of the explosion protection coin. On the other side are the numerous lessons that colleagues have had to learn bitterly without subsequent generations being able to learn from them.
How many explosions have occurred in laboratory refrigerators, with ethers, peroxides, oxidizing agents of all kinds or catalytic decompositions and hydrogen deposits in chemically precipitated metal powders?
Is all this always fully taken into account in the risk assessments?
References
[1] 65th Tutzing Symposium 2017: Perceiving, analyzing and communicating risks - Erosion of Germany as a business location? Protestant Academy Tutzing, Lake Starnberg, 25-27.09.2017
[6] Hasenpusch, W.: Explosive reactions with noble metal compounds, Chem. Ztg. 2 (1987) 57
[7] Hasenpusch, W.: Explosive Reaktions with PGM, lecture and publication, IPMI conference, Brussels, 1987
[8] Hasenpusch, W.: Explosivität von Tetramminmetallnitraten, J. prakt. Chem. 335 (1993), 193
[9] Hasenpusch, W.: Explosive reactions in electroplating, Galvanotechnik; 86 (1995), 64
[10] Hasenpusch, W.: Explosions in refrigerators, Chem. Lab. Biotech., 5 (1995), 212
[11] Knothe, M.; Hasenpusch, W.: Formation of Explosive Chlorine- Nitrogen Compounds during the Reaction of Ammonium Compounds with Chlorine, Inorg. Chem. 35 (1996), 4529
[12] Hasenpusch, W.: Explosive Reactions in Electroplating II, Galvanotechnik; 87 (1996), 87
[13] Hasenpusch, W.: Precautions against fire and explosion, Galvanotechnik 10 (2006)
[14] Hasenpusch, W.: Mixing up chemicals, Galvanotechnik, 2 (2007), 320-327
[15] Hasenpusch, W.: An explosive "sinister" oxidant, ammonium nitrate - a wolf in sheep's clothing, CLB, 01/02 (2013) 18-26
[17] DIN V 14011
[18] Bretherick's Handbook of Reactive Chemical Hazards, 7th edition, hardcover ISBN: 9780123725639; eBook ISBN: 9780080523408, price: 642 US-$ each; Sept. 2006, 2,680 pages
[19] ATEX Directive 2014/34/EU
[20] ATEX Directive 1999/92/EC
[21] Ordinance on Protection against Hazardous Substances (Hazardous Substances Ordinance - GefStoffV) of 26.11. 2010 (Federal Law Gazette I p. 1643) with amendment of 15.11. 2016 (Federal Law Gazette I p. 2549)
[29] IEC 60079-0, standard of the International Electrotechnical Organization (cooperation)
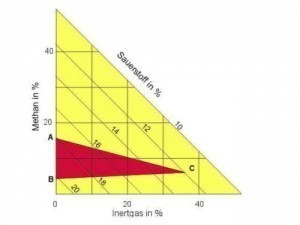 Fig. 14: Explosion area of the gas mixture methane/air/oxygen/inert gas (N2, argon)Primary explosion protection describes measures that prevent the formation of an explosive situation from the outset.
Fig. 14: Explosion area of the gas mixture methane/air/oxygen/inert gas (N2, argon)Primary explosion protection describes measures that prevent the formation of an explosive situation from the outset. Fig 15: Warning sign for a hazardous industrial area (Photo: CEphoto, Uwe Aranas)Preliminary research into the explosive decomposition of chemicals under the influence of various parameters, such as temperature, pressure, atmosphere or highly exothermic reactions, such as polymerization, oxidation, neutralization, also helps to control processes under all circumstances.
Fig 15: Warning sign for a hazardous industrial area (Photo: CEphoto, Uwe Aranas)Preliminary research into the explosive decomposition of chemicals under the influence of various parameters, such as temperature, pressure, atmosphere or highly exothermic reactions, such as polymerization, oxidation, neutralization, also helps to control processes under all circumstances.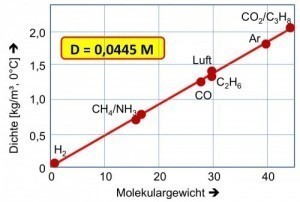 Fig. 16: Densities of gaseous operating fluids in relation to atomic/molecular weight To ensure that extraction systems are used in the right places, the densities of hazardous gases are important in relation to air density, taking into account the temperatures (Fig. 16): Hydrogen, methane and ammonia, for example, are significantly lighter than air at room temperature and can accumulate in less ventilated ceiling areas.
Fig. 16: Densities of gaseous operating fluids in relation to atomic/molecular weight To ensure that extraction systems are used in the right places, the densities of hazardous gases are important in relation to air density, taking into account the temperatures (Fig. 16): Hydrogen, methane and ammonia, for example, are significantly lighter than air at room temperature and can accumulate in less ventilated ceiling areas.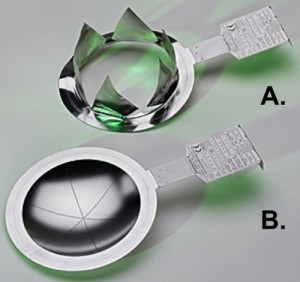 Fig. 17: Rupture disk (bursting diaphragm) adapted to special overpressure after the explosion (A), in the original (B)The following protective measures are possible and must be checked for their special suitability [16]:
Fig. 17: Rupture disk (bursting diaphragm) adapted to special overpressure after the explosion (A), in the original (B)The following protective measures are possible and must be checked for their special suitability [16]:

