Valuable resources and energy can be saved by recycling materials, especially metals. Many components and assemblies, such as the bodies of modern vehicles, contain a variety of different types and grades of material. If recycling by type is not possible due to a lack of analysis options, expensive and high-quality alloying elements are often lost in the general scrap. In this article, the physical measuring principle is first discussed and the functionality of XRF is explained using a mobile analyzer. Using concrete examples from stainless steel recycling, the possible applications and also the application limits are also shown. Finally, other methods that can partially compensate for these limitations are briefly discussed.
1 Physical principles
X-ray fluorescence analysis (XRF) is an effective and relatively simple method for rapid and non-destructive material analysis. This method can be used to determine the composition of a component in terms of the types of elements and their quantity. Therefore, in addition to material recycling and quality control, it also has a firm place in material development.
X-ray fluorescence analysis (XRF) has been used as a routine method for element analysis since the 1960s. Its origins go back to experiments carried out by the two scientists Richard Glocker and Georg Karl von Hevesy in the 1920s. In the last 15 to 20 years in particular, XRF has become increasingly important due to technical advances in the field of electronics and the development of further applications such as layer analysis. The development of hand-held analyzers for mobile use also
tors for mobile use also opened up new areas of application.
It is now the most frequently used method for the qualitative and quantitative determination of elemental composition and is primarily used in the metalworking industry, but also for testing organic and inorganic substances. [1, 2]
X-ray fluorescence analysis makes use of the physical laws of atomic structure. Each element is characterized by the atomic nucleus with a corresponding number of protons and an identical number of electrons on the surrounding shells. The electron shell is divided into different shells or energy levels. These are labeled K, L, M, etc. consecutively from the inside to the outside, whereby K as the shell closest to the nucleus has the lowest energy and the outermost shell has the highest energy [3].
When the test is carried out, primary X-rays generated by an X-ray tube hit the sample to be analyzed. As a result, electrons close to the nucleus are detached from the inner shells of the atom. The resulting gaps are filled by electrons moving in from atomic shells further out. 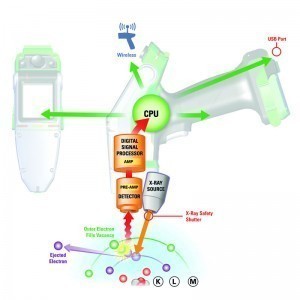 Fig. 1: Schematic representation of how XRF worksIfan electron fallsfrom a higher energy level to a lower one, the energy difference is emitted as X-rays characteristic of the element. This atom-specific characteristic radiation is referred to as X-ray fluorescence. This fluorescence radiation is evaluated by a detector located in the analyzer. By qualitatively determining these element-specific wavelengths, it is possible to determine which elements are present in the sample. By quantitatively determining the intensity of the wavelengths that occur, the concentrations in which the respective elements are present in the sample can be determined. Due to physical laws, the detectable fluorescence decreases sharply for elements with low atomic numbers. This is the reason why light elements such as Mg, Al, Si, P and S are more difficult to measure. The lower detection limit for the element fluorine with atomic number 9 is close to the application.
Fig. 1: Schematic representation of how XRF worksIfan electron fallsfrom a higher energy level to a lower one, the energy difference is emitted as X-rays characteristic of the element. This atom-specific characteristic radiation is referred to as X-ray fluorescence. This fluorescence radiation is evaluated by a detector located in the analyzer. By qualitatively determining these element-specific wavelengths, it is possible to determine which elements are present in the sample. By quantitatively determining the intensity of the wavelengths that occur, the concentrations in which the respective elements are present in the sample can be determined. Due to physical laws, the detectable fluorescence decreases sharply for elements with low atomic numbers. This is the reason why light elements such as Mg, Al, Si, P and S are more difficult to measure. The lower detection limit for the element fluorine with atomic number 9 is close to the application.
The generation of X-ray fluorescence radiation is shown in simplified form in Figure 1. The primary X-ray radiation removes an electron from the L-shell and fills the resulting gap with an electron from the M-shell. This produces the radiation characteristic of the element.
In Figure 2, a simplified example of the spectrum of a high-alloy iron-based material shows the respective intensity as a count rate (counts per second) over the characteristic energies of the elements iron, nickel and chromium. Here, the Kα peaks originate from the electron transitions between the K and L shells and the underlying Kβ displays analogously from the transitions between the K and M shells.
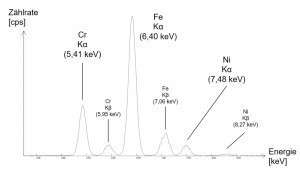 Fig. 2: Simplified spectrum of a high-alloyed iron-based alloyThedepth to which the X-rays can penetrate the material and the depth from which the measurement information originates is determined by the energy of the radiation and its absorption in the sample matrix. The higher the density of a material, the lower the information depth, which is also referred to as the penetration depth. For example, an X-ray tube with a maximum tube voltage of 50 kV, as is often used in hand-held analyzers, can achieve measuring depths of up to 3 mm for copper-based materials. In contrast, this is only 0.6 mm for gold due to its more than double density and a non-linear physical law in the attenuation of X-rays as a function of depth. This fact must be taken into account when carrying out measurements to check, for example, the composition of the component in layers closer to the core. On the other hand, the effect of the density-dependent
Fig. 2: Simplified spectrum of a high-alloyed iron-based alloyThedepth to which the X-rays can penetrate the material and the depth from which the measurement information originates is determined by the energy of the radiation and its absorption in the sample matrix. The higher the density of a material, the lower the information depth, which is also referred to as the penetration depth. For example, an X-ray tube with a maximum tube voltage of 50 kV, as is often used in hand-held analyzers, can achieve measuring depths of up to 3 mm for copper-based materials. In contrast, this is only 0.6 mm for gold due to its more than double density and a non-linear physical law in the attenuation of X-rays as a function of depth. This fact must be taken into account when carrying out measurements to check, for example, the composition of the component in layers closer to the core. On the other hand, the effect of the density-dependent
penetration depth can be used to analyze the thickness of thin coatings, such as those applied in electroplating or cladding. The coating materials and therefore the densities must be known in advance so that their thickness can be determined during the measurement via the respective absorption in a coating. In the case of very thin layers, the respective detection limit of the element used must be taken into account. Due to the high reproducibility of the measurement results and the short measurement times of just a few seconds, this method has become firmly established in non-destructive coating analysis.
2 Use of mobile XRF in the recycling of recyclable materials
The use of mobile handheld spectrometers has opened up a wide range of possible applications for X-ray fluorescence analysis. The typical areas of application of the so-called handheld devices are in quality assurance and mix-up testing, in the recycling of scrap metal and electronic components, in the purchase of precious metals, in RoHS screening, in the determination of heavy metals and halogens in plastics, in soil and ore analysis and in archaeometry.
As this is an X-ray device in accordance with Section 18 (1) No. 1 of the German X-ray Ordinance (RöV), the legal regulations must be observed before operation and an employee must be trained and appointed as a radiation protection officer. In combination with a special sample chamber, a mobile analyzer can be converted very quickly into a stationary system that can be used for the safe measurement of smaller samples in laboratory operations, for example in materials development and also in teaching.
In many cases, it is sufficient to classify materials without carrying out a qualified quantification. This can be the case, for example, when it is necessary to check whether the material used is suitable for the intended application, e.g. whether a steel is resistant to seawater or suitable for welding. Another application could be the determination of the content of alloying elements in scrap in order to decide on its value and further use [2].
Depending on the type and quality of the scrap, the monetary value and recycling potential of the scrap metal are also determined. In principle, the scrap metal arriving at the recycling company is first tested for radioactivity. They are then selected on the basis of the test results and sorted for storage [4].
Even if the experienced producer and user is able to assign an end product to a suitable steel grade based on its recognizable properties, this is often no longer possible with a semi-finished product in the incoming goods inspection or a scrap part. In contrast to stationary analysis, the preparation of the test specimen is relatively simple for scrap sorting. It is advisable to remove coatings or impurities in order to reduce their influence on the determination of light elements in particular. If, for example, a beverage can made of ordinary tinplate is tested, the composition of the coating suggests a titanium content in the steel of almost 5% by weight.
Although the different measuring conditions and degrees of contamination when used in a recycling plant limit the analysis accuracy that can be achieved, in principle, quantitative analysis with mobile devices can achieve analysis accuracies that are comparable with stationary laboratory devices. The detection limits are very strongly dependent on the device, element, accompanying element and matrix, so that a general statement about the measurement accuracy cannot be made. However, the LOD values (Limit of Detection) are much more meaningful, as they range from 10 to 5000 ppm depending on the element. For example, in an iron matrix, the LOD value for Cr is 0.003 wt.%, for Ni 0.020 wt.% and for Al 0.500 wt.%. [5].
-continued-
Literature
[1] Bauch, J.; Rosenkranz, R.: Physical materials diagnostics. Springer Vieweg Verlag, (2017)
[2] Haschke, M.; Flock, J.: X-ray fluorescence analysis in laboratory practice. Wiley-VCH Publishers, (2017)
[3] https://www.analyticon.eu/de/rfa.html
[4] Schlegel, J.: Kleine Stahlkunde - Insights into the world of stainless steels. Rommert Publishing House, (2015)
[5] Thermo Scientific Niton XL3t GOLDD Alloy Analyzers - Elemental Limits of Detection in Titanium/Iron/Copper-based Alloys. Product Data Sheet, (2008)
[6] https://www.edelstahl-rostfrei.de/downloads/iser/MB_821.pdf


