Introduction
The metallization of plastics by electroplating has a wide range of applications. Decorative and ornamental elements as well as operating concepts are often realized with this technology in the automotive, household electronics and furniture industries. This is because the advantages of plastics processing such as production efficiency, free shaping and low density are optimally combined with the advantages of electroplating in a product with high value and durability. Accordingly, these processes can be used to create a wide range of applications and products efficiently and with high quality.
European production plants meet high environmental standards. Nevertheless, the substitution of ingredients and the associated adjustments in the electrolytes pose new challenges, not only in the coating process. Waste water treatment in electroplating must also be adapted to the new processes. In electroplating as an aqueous coating process with several stages of active electrolytes, rinsing processes play an important role in process reliability. The electrolytes in the coating must be separated by rinsing steps and carryover to the next stage must be prevented. Special attention must be paid to their treatment in terms of water requirements, material recovery and, finally, waste water treatment.
The waste water produced during the electroplating of plastic components is environmentally relevant due to its heavy metal content. Due to its composition, it must not be discharged into the municipal wastewater system without treatment. Plastic metallization companies in Germany therefore usually operate their own wastewater treatment plants in which the wastewater is treated accordingly before being discharged.
Since 2013, chromium trioxides (chromium(VI)) and their compounds have been on the ECHA's list of substances subject to authorization [1]. Due to the associated uncertain use horizon in production, sulphate-based chromium(III) electrolytes are increasingly being used as an alternative to chromium(VI)-based electrolytes for the decorative coating of plastic components. These make corresponding adjustments to wastewater treatment necessary. While the rinsing water from hexavalent electrolytes can still be treated by simple hydroxide precipitation, this is no longer possible with trivalent electrolyte rinses. For this reason, the BIA Group, in cooperation with Münster University of Applied Sciences and chemical manufacturers, has tested various treatment options for use under series production conditions.
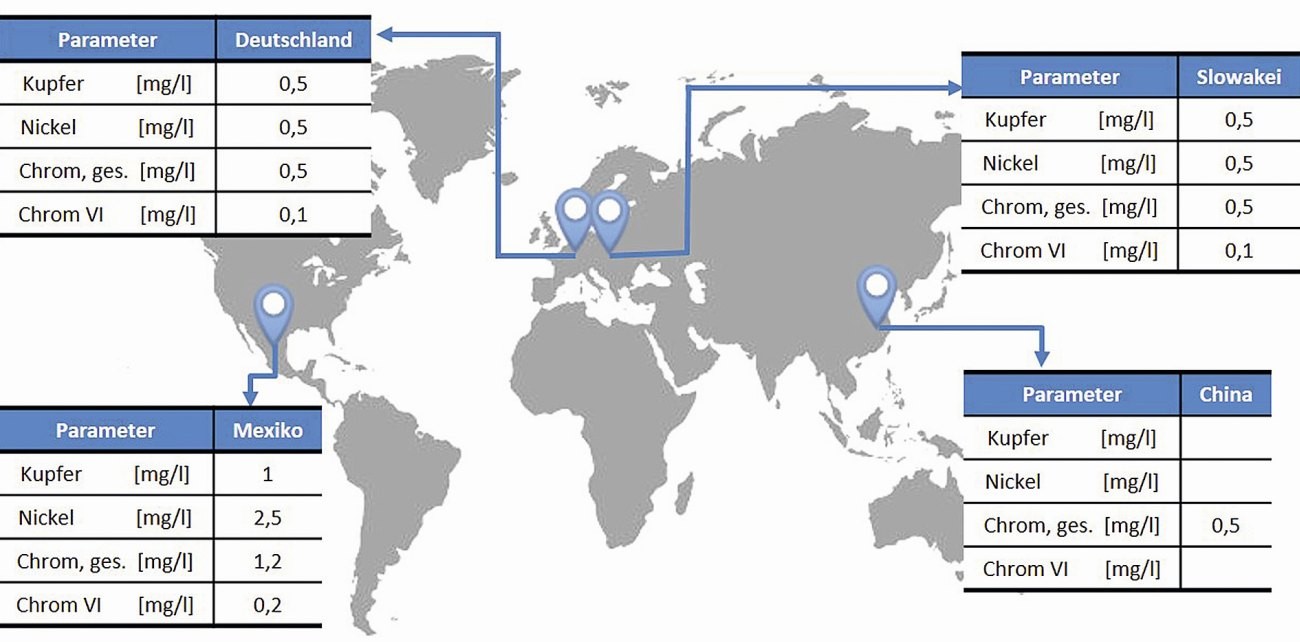 Fig. 1: Discharge values at four BIA Kunststoff- und Galvanotechnik sites
Fig. 1: Discharge values at four BIA Kunststoff- und Galvanotechnik sites
Legal requirements
Certain legal requirements must be met for the discharge of wastewater. In Germany, these are laid down in the Water Resources Act (WHG). According to §58 WHG [2], a permit from the local authority is required for discharge into public wastewater systems (indirect discharge), which is usually issued as a statutory permit. One of the requirements for obtaining a permit is the operation of a wastewater treatment plant. According to §57 WHG [2], the discharge of wastewater into bodies of water (direct discharge) may only be permitted if the quantity and harmfulness of the wastewater is kept to a minimum through state-of-the-art treatment. The requirements to be met according to the state of the art depending on the source of the wastewater are described in the Federal Wastewater Ordinance (AbwV). For metalworking and processing companies, the limit values set out in Annex 40 of the AbwV apply. This also contains limit values for mixing with other wastewater. These in turn must be complied with by both direct and indirect dischargers. In some cases, the specifications in the WHG are specified by local legislation. However, this must then be considered on a site-specific basis.
According to the WHG, compliance with the discharge limits applicable to the respective company must be monitored by regular sampling by experts. There are similar legal limits worldwide for the discharge of wastewater containing heavy metals from electroplating plants, as shown in Figure 1, which shows a selection of the legal discharge limits at BIA sites worldwide.
The limit values shown are very low and in the range of trace elements. Accordingly, a mature and proven technology for treating the rinse water is necessary to ensure that these limits are clearly undercut. Accordingly, it is important to validate the wastewater treatment in advance for new processes and, if necessary, to develop it as a process. The composition of the electrolytes is of course the most important factor here.
Comparison between sulphate-based chromium(III) and chromium(VI) electrolytes
Chromium-containing electrolytes are used in plastic metallization for the final step of the coating, the so-called chrome finish. Chromium(III)-containing electrolytes are more complex to handle in terms of process engineering than chromium(VI)-based electrolytes. The composition of the electrolytes is mainly based on chromium(III) sulphate, boric acid, potassium hydroxide, organic complexing agents and other additives (including wetting agents). The chromium is complexed in the chromium(III) electrolyte. This represents a major difference to chromium(VI) electrolytes, in which chromium is present as an anion. While the chromate anions in the acidic medium can be handled stably in the processes, this is not the case with trivalent chromium compounds. Here, a chromium(III) complex must be formed in order to keep the chromium in solution. The complexing agents contained stabilize the electrolyte and ensure controlled separation of the chromium, but also enter the wastewater, where their complex-forming properties are not desirable.
Basics of wastewater treatment in plastic metallization
In series production with electroplating, the active baths are separated by rinsing in order to ensure high quality and avoid problems caused by contamination and carryover. In electroplating, appropriate rinsing techniques such as cascade rinsing or ion exchanger rinsing are used to ensure the most water-efficient production possible. With cascade rinsing, the first rinse after the active bath is much more concentrated compared to a pure stationary rinse, which makes partial recirculation into the active bath possible. This reduces the contamination of the rinse water. Ion exchanger rinses are generally used as the last rinsing step. The water in these rinses is filtered using an ion exchange resin, which keeps the rinses clean and saves further rinsing steps. In terms of plant technology, the batch treatment principle has become established for waste water systems in plastic metallization.
The waste water resulting from the electroplating processes is not only of oxytoxic and ecotoxic significance due to the substances contained in the electrolytes, but it is also economically relevant as it contains high-purity recyclable materials. The electrolytes commonly used in coatings for the sanitary and automotive industries mainly contain heavy metals, phosphate, phosphite, sulphate, chloride and ammonium as wastewater-relevant substances.
In the case of the trivalent electrolytes newly introduced in production, the focus can be placed on the complex-bound heavy metal components. Heavy metals are usually removed from wastewater by hydroxide precipitation. Depending on the oxidative state, an additional reduction step in an acidic environment may be necessary beforehand. The hexavalent oxidation states of chromium could still be easily treated in this way, but the complexed trivalent chromium must be considered differently.
If the heavy metals are complexed, i.e. in stronger complexes than the aqua complexes, these are precipitated using organosulphides or the solution is treated using AOP (Advanced Oxidation Process) methods before hydroxide precipitation. Organosulphides are used to convert the metal complexes into insoluble products that can be settled in a further step and ultimately removed as solids. The advantages of organosulphides are the short treatment time and the low residual metal content. Their contribution to possible water toxicity is a disadvantage [3]. Advanced oxidation processes (AOP) have the advantage that the use of chemicals is low and no harmful by-products are produced. The photocatalytic reaction is explained in more detail in the section on the fourth treatment test. The disadvantages of this method compared to sulphide precipitation are the high energy consumption and the longer treatment time.
Depending on the load of the wastewater and the local discharge limits, an ammonium stripping process may be necessary after the precipitation process or a reduction of the phosphate and sulphate load (e.g. the use of calcium hydroxide) may be necessary during the precipitation process. The heavy metal hydroxide sludge resulting from precipitation should be as pure or concentrated as possible so that recycling and thus recovery of the valuable materials it contains is economical.
Treatment trials with wastewater containing chromium(III)
Various methods have been tested for the treatment of wastewater from sulphate-based chromium(III) electrolytes:
- Simple hydroxide precipitation (classic method)
- Recomplexation in acid using iron(III) chloride and subsequent hydroxide precipitation
- Method from a chemical supplier, which is essentially based on chemical oxidation of the electrolyte components, a reduction step and finally hydroxide precipitation
- UV peroxide method with subsequent reduction step and hydroxide precipitation.
To ensure that the tests remain comparable, a simulated rinse with 10 % of the electrolyte concentration and a treatment volume of one liter was used for all tests. The rinse concentration of 10 % of the electrolyte concentration was chosen as this corresponds to an average concentration when using stand rinses and discarding the rinses in production environments. Chromium(III) electrolytes from two different suppliers (electrolyte 1 and electrolyte 2) were tested in all experiments.
The treatment tests are described below and the results are presented. A reduction of the chromium content by 99.9 % is sufficient to achieve the legally required limit value (in Germany). Finally, the different methods are compared (see Table 1).
- Addition of NaOH
- Addition of Ca(OH)2
- Addition of NaOH
- Addition of flocculant
- Filtration
Conventional hydroxide precipitation
A conventional hydroxide precipitation using calcium hydroxide and sodium hydroxide was carried out as a reference method. By adding alkaline solutions and precipitation aids, the heavy metals should precipitate out of the solution as hydroxides. The partial use of calcium hydroxide suppresses the amphoteric character of the chromium even at higher pH values, as the calcium ions form, among other things, poorly soluble metal compounds. Finally, the pH value is raised to a value of 10.5 with a sodium hydroxide solution and the solution is filtered off after sedimentation.
Figure 2 shows the process sequence of the precipitation reaction and the separated phases after the sedimentation step.
After treatment of the chromium(III) wastewater by hydroxide precipitation, a reduction in the chromium content of 90 % and a concentration after treatment of 84 mg/l was found for electrolyte 2. Electrolyte 1 could not be brought within the limit values either. The method is not sufficient to comply with the legal limits. Even an increase in the concentration of the additions could not achieve any improvement.
Recomplexing in the acid using iron(III) chloride and subsequent hydroxide precipitation
The second method tested was hydroxide precipitation with the prior addition of iron(III) chloride. After the first series of tests and the high residual chromium content, it was assumed that the chromium complexes were still present in the solution and therefore the chromium could not be precipitated. The addition of iron(III) chloride leads to partial recomplexation, which should make the chromium easier to precipitate.
Figure 3 shows an improvement in the color of the wastewater sample compared to normal hydroxide precipitation. However, higher sludge formation is also to be expected, which is detrimental to the disposal of the solids.
After treatment of the chromium(III) wastewater, a reduction in the chromium content of 97-98.5 % and a concentration of 24.8-14 mg/L chromium (total) was found. This confirms the assumption that the complex-bound components of chromium are problematic in treatment. By recomplexing with iron, a significantly stronger precipitation and reduction of the chromium content could be achieved here. However, this method is not sufficient to comply with the legal limits. In addition, the addition of iron results in larger quantities of sludge in the sedimentation process, which in turn means high costs in the process sequence and is reflected in higher disposal costs.
- Addition of Fe(III)CI3 solution (60 ml/l)
- Addition of NaOH
- Addition of Ca(OH)2
- Addition of NaOH
- Addition of flocculant
- Filtration
Oxidation and reduction of the electrolyte components with final hydroxide precipitation
The third treatment combines oxidation and subsequent reduction of the electrolyte components with final precipitation under specific pH conditions. For this purpose, the wastewater sample was first strongly acidified. Strong oxidizing agents (including manganates) were then added. After maintaining the redox potential for several hours, the reduction step was carried out using iron(II) salt, among other things. The detoxification of the chromate-containing wastewater was determined using the redox potential. Finally, the pH value was raised and the sedimentation process was supported with precipitants and additional flocculants. After treatment of the chromium(III) wastewater, a reduction in the chromium content of 98.6 - 99.3 % and a concentration of 11-7 mg/L chromium (total) were measured. The advantages of this test were lower sludge formation and a significantly greater reduction in the heavy metal concentration compared to methods 1 and 2. The disadvantages were the very long reaction times between the respective steps, the high chemical consumption and the quantity of the various chemicals required. The high number of process steps as well as the influence of the reaction time and the quantities of additional reaction chemicals used show a good effect. However, it has not been possible to achieve safe levels below the limit values. The process can be functional for lower concentrations, but safety in production is not achieved. In addition, the expenses for process time, reaction steps and chemistry are problematic from an economic point of view.
-will be continued
- Acidification of the solution
- Addition of oxidizing agent and iron salts
- Addition of reducing agent
- Addition of Ca(OH)2
- Addition of Na-Al salts
- Addition of flocculant
- Filtration
Literature
[1] European Chemicals Agency (ECHA): Regulation (EC) No 1907/2006. URL https://echa.europa.eu/de/authorisation-list - Review date 2021-01-11
[2] Federal Republic of Germany: Law on the organization of the water balance - Water Resources Act (as amended on 31. 7. 2009). URL https://www.bgbl.de/xaver/bgbl/start.xav?startbk=Bundesanzeiger_BGBl&jumpTo=bgbl109s2585.pdf#__bgbl__%2F%2F*%5B%40attr_id%3D%27bgbl109s2585.pdf%27%5D__1610358307085 – Überprüfungsdatum 2021-01-11
[3] Gartiser, S.; Hafner, C.; Hercher, C.; Kronenberger-Schäfer, K.; Paschke, A.: Whole effluent assessment of industrial wastewater for determination of BAT compliance, Part 2: metal surface treatment industry (5)
[4] Peñafiel Ayala, R.: Photocatalytische Behandlung von biologisch schwer abbaubauren Wasserverunreinigung mit Titandioxid und simuliertem Sonnenlicht, Berlin, Technische Universität Berlin. approved dissertation. 2002











