The metallization of plastics by electroplating has a wide range of applications. Decorative and ornamental elements as well as operating concepts are often realized with this technology in the automotive, household electronics and furniture industries.
UV peroxide treatment
Finally, UV-H2O2 oxidation has been tested as a pre-treatment method for chromium(III)-containing waste water. For this purpose, a test system (see Fig. 5) with a 15 L storage tank was used and samples were taken at various intervals, which were then treated by precipitation reaction.
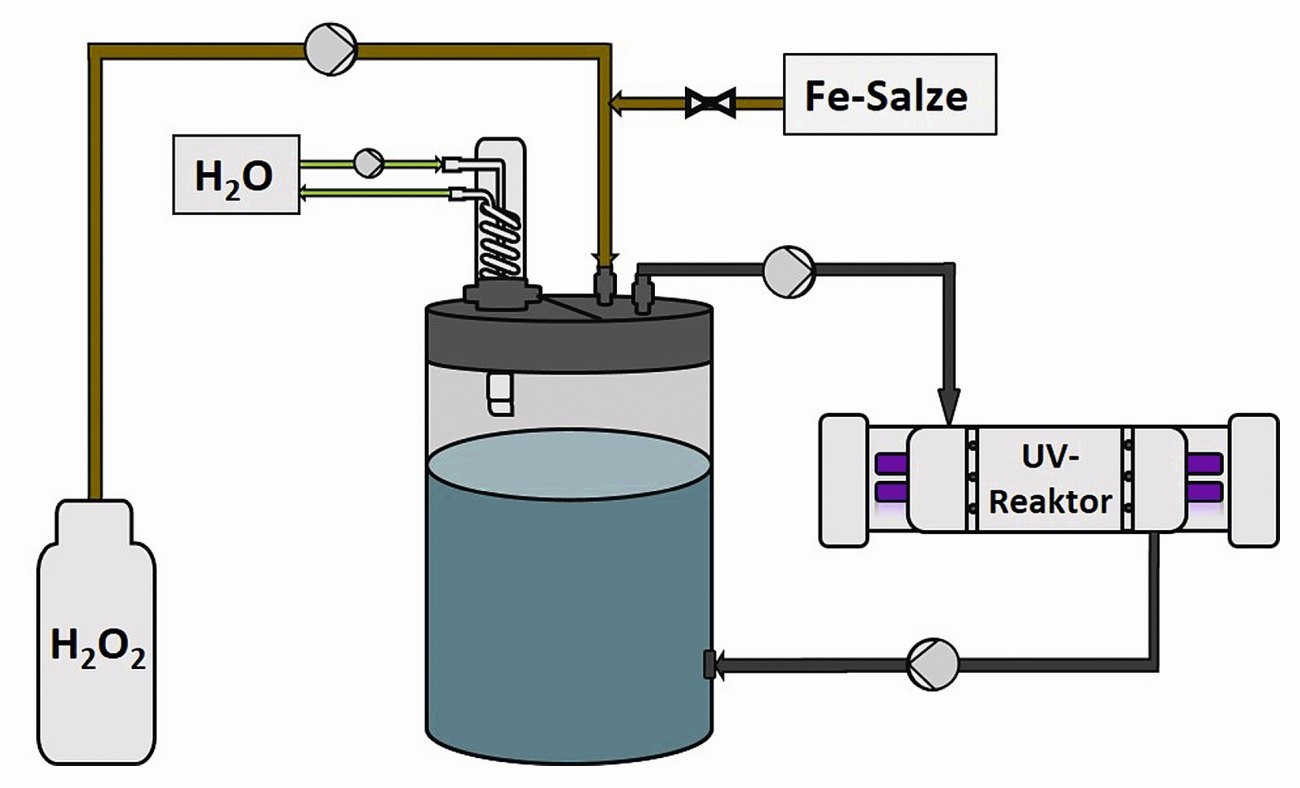 Fig. 5: Schematic representation of a UV pilot plant
Fig. 5: Schematic representation of a UV pilot plant
The mechanism of UV peroxide oxidation is based on a complex radical chain reaction. Hydrogen peroxide is homolytically cleaved by the UV light and hydroxyl radicals are formed as a result (see reaction diagram 1). These strong oxidizing agents react with the chromium(III) complexes and the free complexing agents. Iron salts catalyze the reaction.
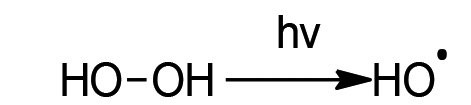 Reaction Eq. 1: Homolytic cleavage of hydrogen peroxide
Reaction Eq. 1: Homolytic cleavage of hydrogen peroxide
A Fenton reagent (consisting of Fe3+ and H2O2) was used for this in the experiment. H2O2 is homolytically cleaved by UV irradiation and at the same time iron(III) is reduced to iron(II). The resulting OH radicals are very important oxidizing agents and can react quickly and unspecifically with almost all organic compounds. In combination with oxygen molecules, the organic compounds are mineralized and ultimately completely oxidized [4].
The resulting chromium and organic compounds in the oxidized state must first be neutralized. The neutralization reaction was carried out with a suitable reducing agent and finally the heavy metal hydroxides were sedimented as sludge in the alkaline range using precipitants.
After treatment of the chromium(III) wastewater, a reduction in the chromium content of 99.99% and a concentration of 0.04-0.02 mg/L chromium (total) was achieved. The method is therefore suitable for both electrolytes tested in order to comply with the legal limits.
- Addition of reducing agent
- Addition of NaOH
- Addition of Ca(OH)2
- Addition of NaAIO2
- Addition of flocculant
- Filtration
Fig. 6: Treatment of a wastewater sample containing chromium(III) by means of hydroxide precipitation after photochemical oxidation
Comparison of the treatment methods
Rapid cuvette tests (UV measurement) and ICP-OES measurements were carried out to determine the chromium concentrations. Chromium concentrations were analyzed both at the beginning before treatment and after each treatment method. The values determined by ICP-OES measurement are listed in Table 1 and the percentage reduction from the various processes is shown in Figure 7.
|
Process |
Electrolyte |
Chromium initial concentration [mg/l] |
Final chromium concentration [mg/l] |
Percentage reduction [%] |
|
I |
1 |
1.636 |
570,4 |
65,1 |
|
2 |
892,2 |
83,8 |
90,6 |
|
|
II |
1 |
816,9 |
24,8 |
97,0 |
|
2 |
949,4 |
14,3 |
98,5 |
|
|
III |
1 |
798,9 |
11,0 |
98,6 |
|
2 |
949,4 |
6,9 |
99,3 |
|
|
IV |
1 |
854,5 |
0,04 |
99,99 |
|
2 |
874,4 |
0,02 |
99,99 |
Table 1 shows that the higher the initial concentration of chromium(III)-containing wastewater, the more difficult it is to treat using conventional methods. With an initial chromium concentration of 1,636 mg/l, only a chromium reduction of 65 % was achieved after hydroxide precipitation. This value is so far below the percentage reduction of the other tests that it was no longer taken into account for the presentation of the results in Figure 7 .
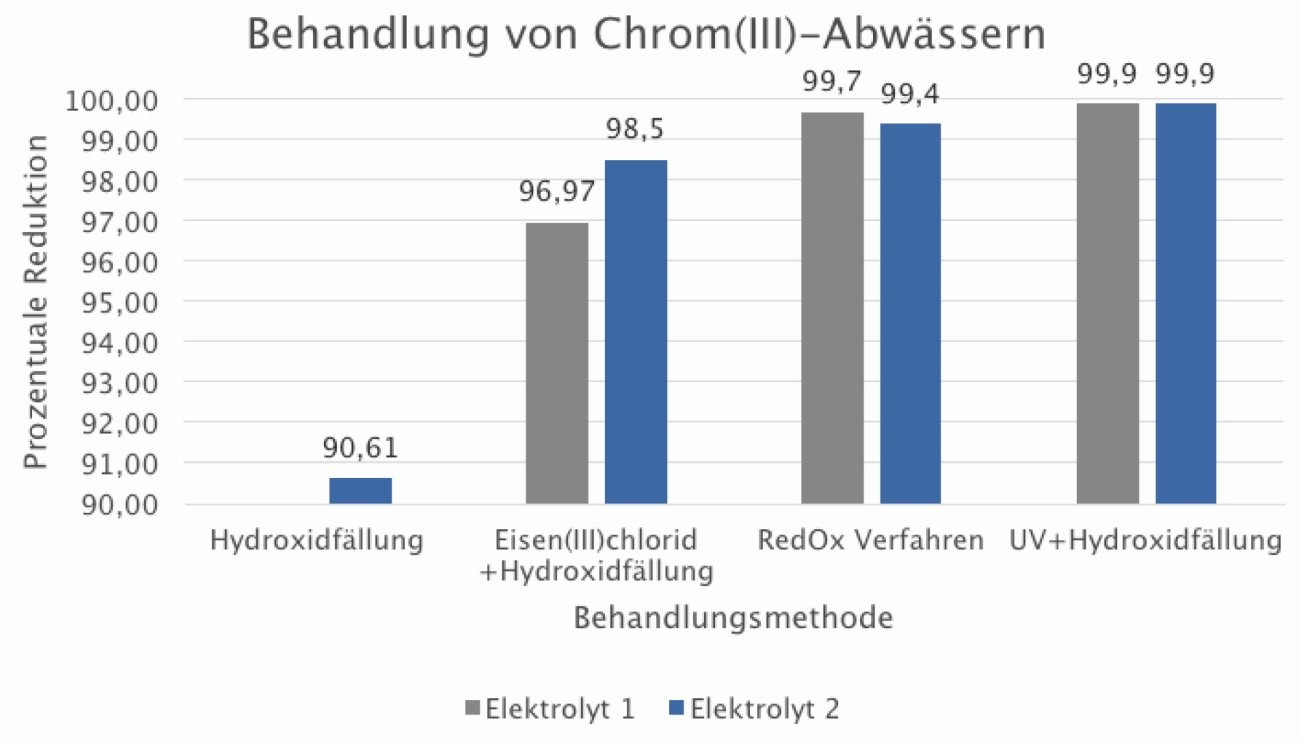 Fig. 7: Overview of the percentage reduction of four different processes for chromium(III) wastewater treatment
Fig. 7: Overview of the percentage reduction of four different processes for chromium(III) wastewater treatment
The results in Figure 7 show the percentage reduction in the treatment of 1 liter of wastewater sample with a pH value of 10.5 according to the four methods. The following conclusions can be drawn from this: Hydroxide precipitation works as an aid to reduce the concentration of chromium, but this is not sufficient for the legal requirements of AbwV Annex 40. The process using oxidizing and reducing agents resulted in a significant reduction in chromium concentrations compared to hydroxide precipitation. Despite long reaction times and different chemicals, the legally required chromium limit value was not reached. This means that even these improvements cannot be used in production in a reliable and economical manner compared to conventional precipitation. This applies at least for electrolyte concentrations in the rinse of approx. 10 %. At lower concentrations, treatment according to process 3, as well as process 2, may be sufficient.
Legally compliant results
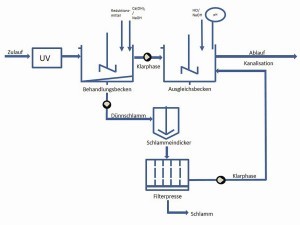 Fig. 8: UV treatment and chemical-mechanical precipitation reaction with subsequent fine purification step of the treated wastewater for discharge into the sewer systemTheUV process with hydrogen peroxide and iron(III) salts showed a clearly better result compared to the first tests. After a treatment time of just 24 hours, the complexes could be broken down to such an extent that legally compliant chromium concentrations were achieved in the clear phase after the treatment time.
Fig. 8: UV treatment and chemical-mechanical precipitation reaction with subsequent fine purification step of the treated wastewater for discharge into the sewer systemTheUV process with hydrogen peroxide and iron(III) salts showed a clearly better result compared to the first tests. After a treatment time of just 24 hours, the complexes could be broken down to such an extent that legally compliant chromium concentrations were achieved in the clear phase after the treatment time.
Figure 8 shows a schematic of the process for a possible treatment of wastewater containing chromium(III) with a UV system and subsequent neutralization/precipitation reaction.
Finally, the clear phase is drawn off and the sludge phase is passed through a chamber filter press. The sludge is collected for disposal and the press outlet and any clear phase are discharged into the sewage system after passing the final filtration.
Conclusion:
The analyses presented show that a very reliable rinse water treatment of trivalent chrome electrolytes can be implemented. This means that a change in coating from hexavalent to trivalent electrolytes does not represent a technical risk in this area, even though the economic costs of the processes are higher than those of the current processes. In terms of the chemicals used, the resulting amount of sludge and the reduction in heavy metals achieved, the UV peroxide process is currently a very good choice for production. Of course, investment costs and operating costs must also be considered here, but safe and fast treatment is possible with this process. However, classic precipitation and the use of chemical oxidizing agents offer potential for further developments. If weaker complexes can be used in the electrolytes, these processes also offer an interesting approach.
[1] European Chemicals Agency (ECHA): Regulation (EC) No. 1907/2006, URL https://echa.europa.eu/de/authorisation-list - Review date 2021-01-11
[2] Federal Republic of Germany: Law on the organization of the water balance - Water Resources Act (as amended on 31. 7. 2009), URL https://www.bgbl.de/xaver/bgbl/start.xav?startbk=Bundesanzeiger_BGBl&jumpTo=bgbl109s2585.pdf#__bgbl__%2F%2F*%5B%40attr_id%3D%27bgbl109s2585.pdf%27%5D_1610358307085 - Review date 2021-01-11
[3] Gartiser, S.; Hafner, C.; Hercher, C.; Kronenberger-Schäfer, K.; Paschke, A.: Whole effluent assessment of industrial wastewater for determination of BAT compliance, Part 2: metal surface treatment industry (5)
[4] Peñafiel Ayala, R.: Photocatalytische Behandlung von biologisch schwer abbaubauren Wasserverunreinigung mit Titandioxid und simuliertem Sonnenlicht, Berlin, Technische Universität Berlin, genehmigte Dissertation, 2002






