- Part 1 - From bauxite mining to the raw metal
Aluminium has been known since the beginning of industrialization. The light metal, which is the third most common metal in the earth's crust when bound in ore, can now be found in more and more product areas. Since the millennium, the proportion of aluminum, which is around three times lighter than iron, has grown significantly as part of downsizing, for example in the automotive industry. In a multi-part series, Kristin Pippig-Schmid presents the metal in detail, from production and coating to future forecasts.
Compared to other metals, the history of aluminum is still very young. Less than 200 years ago, the first ingot of pure aluminum was presented at the world exhibition in Paris in 1855 (Fig. 1). Emperor Napoleon only used aluminum cutlery on very special occasions, as it was traded like gold at the time due to its complex production from bauxite. Fig. 1: Here in the Palais de l'Industrie, the first ingot of pure aluminum was presented at the 1855 World's Fair
Fig. 1: Here in the Palais de l'Industrie, the first ingot of pure aluminum was presented at the 1855 World's Fair
Today, aluminum has found its way into many areas of our lives. Thanks to intensive research and countless application developments, we have succeeded in making the light metal aluminum "salon-compatible". It can be used in a variety of ways and has become an integral part of our everyday lives. Every one of us has already come into contact with this versatile material. When opening a beverage can, travelling by plane and car or on a training ride on a mountain bike, intensive industrial developments began in the 19th century to produce metallic aluminum on an industrial scale. The breakthrough came at the end of the 19th century with the development of fused-salt electrolysis of aluminum oxide (Al2O3) by Paul L.T. Hérould and Charles M. Hall. For economic and process engineering reasons, the Austrian chemist K.J. Bayer researched the digestion of bauxite to Al2O3 with caustic soda at almost the same time. This process, known as the "Bayer process", is still in industrial use today.
Bauxite contains aluminum oxide
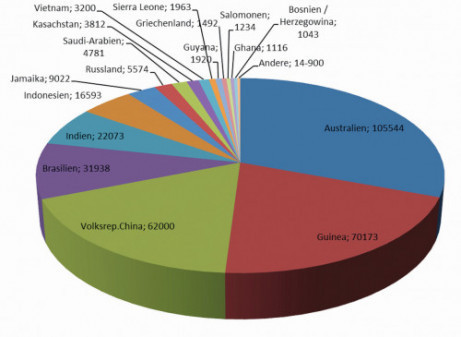
At around 8%, aluminum is the third most common element in the earth's crust. However, aluminum does not occur as a metal in the earth's crust, but only in bound form. Bauxite ore, a reddish-colored sedimentary rock, contains a large proportion of aluminum oxide, which is the starting point for aluminum extraction (Fig. 2). Bauxite is a residue rock formed by the weathering of various rocks. The world's best-known bauxite deposits are found in subtropical and tropical locations such as Guinea, Central and South America, China, India and Australia (Fig. 3). The aluminum content of bauxite deposits varies depending on the mining area and can be between 40-55 %. The aluminum content in bauxite has a direct influence on the amount of energy required for production. The higher the aluminum content in the bauxite, the better the yield and cost calculation.
Composition of bauxite:
Bauxite is an aluminum ore that has approximately the following composition for aluminum extraction:
- Aluminum in the form of aluminum hydroxides (Al(OH)3, AlO(OH))
- Iron oxide Fe2O3
- Titanium oxide TiO2
- Silicon oxide SiO2
Turning bauxite into alumina - the Bayer process
In the Bayer process, aluminum is separated as a hydroxide compound from the other minerals found in bauxite. To do this, the bauxite ore is first crushed and ground. The ore is then mixed with hot caustic soda and lime in pressurized containers and broken down under pressure. The temperatures for this process are between 120 and 300 °C and at pressures of up to 200 bar. The parameters used depend on the type of bauxite and the plant technology.
Aluminum hydroxide is largely soluble in caustic soda. It is present in the solution as a sodium aluminate complex [Na(Al(OH)4)]. The solution is filtered and separated from the undissolved components. The undissolved part is washed and dried and forms the so-called red mud. The iron-containing components lead to the characteristic red coloration. This must be stored safely and in an environmentally friendly manner in red mud landfills or sludge ponds. Approximately 0.5 to 0.7 tons of red mud are produced during the production of one ton of alumina.
Red mud - a hazardous by-product
This is pumped into large, diked and sealed landfills. The red mud landfill is consolidated by natural drying and can be recultivated with native plants. The measures take several years, but they make it possible to restore a near-natural landscape.
However, the storage of red mud is also a controversial issue, as it is stored openly in large sludge landfills. If the landfills are not properly sealed, accidents can happen, as in 2010 when the dam of a landfill basin of the aluminum smelter MAL AG (Magyar Alumínium) in Ajka, Hungary, burst. Around one million cubic meters of corrosive sludge containing heavy metals escaped. Several people died or were injured by the mud flood. Over 40 square kilometers of flora and fauna were severely affected.
Molten flux electrolysis according to Hall-Héroult
The cooled sodium aluminate solution is inoculated and precipitated using solid aluminum hydroxide. After filtration, the liquid phase (caustic soda) is evaporated and returned to the digestion process. This creates a cycle for reusing the caustic soda. The precipitated aluminum hydroxide is washed with water and calcined in rotary kilns at temperatures of 1200 to 1300 °C to produce aluminum oxide. By splitting off the water content, the aluminum hydrate is converted into aluminum oxide.
2 Al(OH)3 → Al2O3 + 3 H2O
The aluminum oxide, known as alumina, has a purity of 99.5% and is the starting material for the production of metallic aluminum. Alumina is reduced to aluminum in an electrolytic process. As alumina (Al2O3) has a melting point of > 2000 °C, the alumina is placed in a cryolite melting bath. Cryolite Na3AlF6 and the fluorine compounds used, aluminum fluoride AlF3 and calcium fluoride CaF2, have the property of reducing the melting point of the aluminum oxide from over 2000 to 950 to 970 °C. The added salts also increase the conductivity of the melt.
The electrolytic cell consists of a refractory-lined steel tank. The additional graphite or carbon lining serves as a cathode. The anodes usually consist of graphite cylinders and are immersed in the molten metal. The aluminum oxide (alumina) is present in the melt as Al3+ and O2- ions. At the cathode, aluminum ions are reduced to metallic aluminum during the molten flux electrolysis. The aluminum is deposited at the bottom of the cell and is regularly extracted under vacuum. Alumina is added to keep the deposition process running. The deposited liquid aluminum has a purity of > 99.5 % and can be increased to 99.99 % by further electrochemical processes.
At the graphite anode, the oxide ions are oxidized to oxygen and react with the carbon anode to form carbon monoxide and carbon dioxide.
to form carbon monoxide and carbon dioxide. This reaction mechanism consumes the graphite anodes and they have to be replaced regularly.
The entire redox reaction of aluminum production (I) can be described in the partial reactions that take place at the cathode (II) and anode (III):
(I) Overall reaction
2 Al2O3 + 3 C → 4 Al + 3CO2
(II)Reduction at the cathode
4 Al3+ + 12 e- → 4 Al
(III)Oxidation at the anode
3 C + 6 O2 → 3CO2 + 12 e-
The driving force of the process is the conversion of electrons, which is set in motion by applying a DC voltage. The applied voltage is relatively low at 4 to 6 volts, but the current flow is very high at up to 300,000 amperes.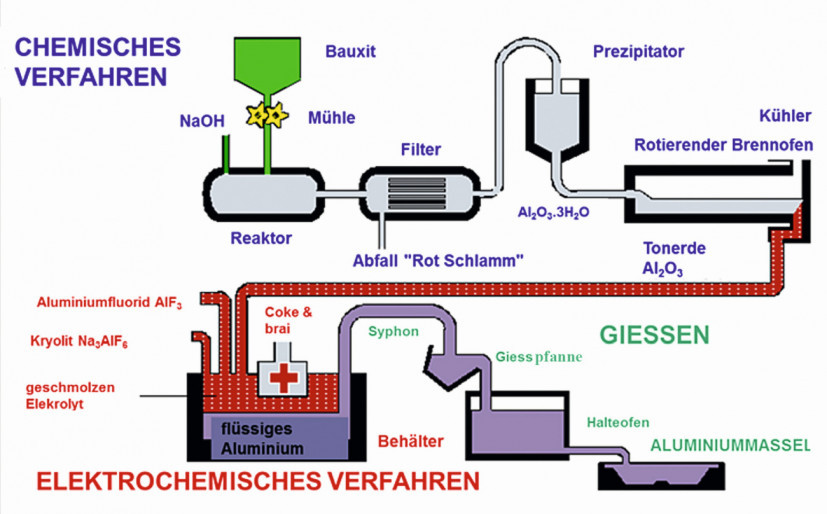 Fig. 4: Overall sequence of aluminium production with Bayer process and smelting electrolysis/ Graphic: Constellium
Fig. 4: Overall sequence of aluminium production with Bayer process and smelting electrolysis/ Graphic: Constellium
The diagram in Figure 4 shows a schematic overview of the overall aluminum production process. The chemical process (Bayer process) in the upper half of the picture shows the bauxite digestion using caustic soda and the resulting alumina production. The alumina is the starting material for aluminum production using the electrochemical process (smelting electrolysis) in the lower half of the picture. The diagram shows that this is a complex multi-stage process that also requires a high energy input.
High demand for raw materials
Four tons of bauxite and two tons of alumina are required to produce one ton of aluminium (Fig. 5). In addition, around 4 kilograms of cryolite and up to 20 tons of aluminium fluoride are required. The procurement of cryolite, which is rarely found in nature, is very expensive, which is why the flux is produced from hexafluorosilicic acid. The flux is used again and again in the cycle, which also explains the small quantities. The waste gases produced during the process, such as carbon dioxide and toxic carbon monoxide, which contain very aggressive fluorine compounds, are extracted in a targeted manner. The resulting fluorine compounds are adsorbed with the introduced alumina and fed back into the cycle.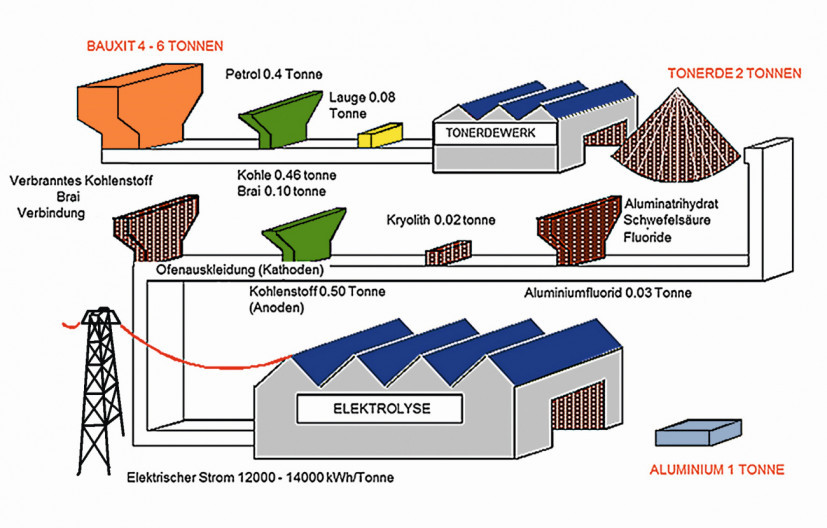 Fig. 5: The production of primary aluminum is complex and resource-intensive, which makes recycling in this area particularly important / Graphic: Constellium
Fig. 5: The production of primary aluminum is complex and resource-intensive, which makes recycling in this area particularly important / Graphic: Constellium
Aluminum alloys
The aluminum is cast into small ingots. In the aluminum plants, the desired aluminum alloys are produced by adding various minerals and metals such as magnesium, silicon, zinc or copper. These are cast into rolling ingots or extrusion billets and then further processed into plates, sheets or profiles. The production of primary aluminum requires a great deal of energy. Secondary aluminum produced from the recycling of aluminum is therefore worthwhile due to the significantly lower use of resources.
Despite the very energy-intensive processes involved in producing metallic aluminum, the countless possible applications combined with the fantastic properties of this light metal justify this effort.
The next part of our aluminum series will take a closer look at aluminum alloys and the hot and cold working of this versatile light metal.