- Part 4 - The process in detail, technical aspects, applications / Continued from Galvanotechnik 1/2024
The anodizing process (electrical oxidation of aluminium), also known as the anodizing process, has undergone a remarkable development in the course of its history: From its beginnings as a decorative process to an indispensable process in various industries. Ongoing research and development have helped to refine the process and make it a key method for improving the properties of aluminum surfaces.
The anodizing process was discovered in the late 19th century when researchers were investigating the electrochemical properties of aluminum. It was found that treating aluminum with an electric current and certain acids led to the formation of an oxide layer. Over the course of the 20th century, anodizing processes were further improved and refined to optimize the quality of the coatings produced. This included the development of specific electrolytes, optimization of process parameters and improvement of control methods.
The layer structure
Anodizing is an electrochemical process that has a wide range of applications. After suitable chemical pre-treatment, the parts to be oxidized are immersed in an electrolyte, usually sulphuric acid or mixed electrolytes, and contacted at a current source. The component forms the anode. The cathodes in the electrolyte bath are usually made of aluminum or titanium. A voltage is applied and a current flows through the electrolyte, which causes oxygen to develop at the anode as a result of electrolysis. The oxygen produced on the aluminum surface (anode) reacts with the aluminum to form an increasingly thick oxide layer with a regular pore structure during the process. The oxide layer essentially consists of two main layers:
The barrier layer (inner layer)
The barrier layer is formed first on the aluminum surface and forms a dense, non-porous aluminum oxide layer. This barrier layer, also known as the barrier layer, depends on the process parameters and is only a few nanometers thick. Due to the dense oxide layer, it acts as a protective barrier for the underlying aluminum. This dense layer provides high corrosion resistance and improves the durability of the anodized surface by preventing the penetration of moisture, chemicals or other harmful substances.
For highly corrosive aluminum components, various developments have shown that barrier layer thickening is possible. Barrier layer thicknesses can be subsequently increased through targeted parameter control and electrolyte selection. This makes it possible to strengthen the barrier layer and increase the properties such as corrosion and chemical resistance.
Porous layer (outer layer)
The barrier layer is repeatedly "penetrated" by the applied stress and a porous outer layer is created. The barrier layer is closed again and again. The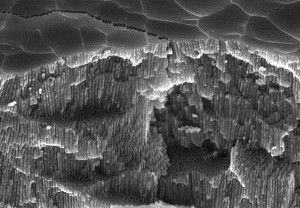 Fig. 1: Fracture surface view of the pore structure of an anodized layer GS- process oxide layer built up on the pore base is porous, with the pores being in the nanometer range. This constantly repeating process causes the aluminum oxide layer to build up from the inside out. Depending on the process conditions, the anodic oxide layer, which is firmly anchored to the base material, grows inwards and outwards to a certain extent, which must be taken into account when defining tolerances for precision parts. With standard sulphuric acid anodizing, it is assumed that 2/3 grows inwards and 1/3 outwards. This means that with a layer thickness of 20µm, only about 7µm grow outwards. In the case of hard anodizing (sulphur oxalic acid), half of the oxide layer is expected to grow inwards and half outwards.
Fig. 1: Fracture surface view of the pore structure of an anodized layer GS- process oxide layer built up on the pore base is porous, with the pores being in the nanometer range. This constantly repeating process causes the aluminum oxide layer to build up from the inside out. Depending on the process conditions, the anodic oxide layer, which is firmly anchored to the base material, grows inwards and outwards to a certain extent, which must be taken into account when defining tolerances for precision parts. With standard sulphuric acid anodizing, it is assumed that 2/3 grows inwards and 1/3 outwards. This means that with a layer thickness of 20µm, only about 7µm grow outwards. In the case of hard anodizing (sulphur oxalic acid), half of the oxide layer is expected to grow inwards and half outwards.
The pores are characteristic of anodized surfaces and can be controlled in their density and size, depending on the requirements of the application. Aluminum oxide layers are transparent and are referred to as colorless without additional coloring. However, the oxide layer pores can also be colored. In this case, the pores are filled with colored pigments or metal salts to achieve a wide range of colors (Fig. 1).
The process steps
The anodizing process according to DIN 17611 includes various treatment stages (Fig. 2).
 Fig. 2: Diagram of the anodizing process
Fig. 2: Diagram of the anodizing process
Mechanical pre-treatment
Components can be ground and/or brushed before anodizing. This pre-treatment is used either for mechanical pre-cleaning of components, but primarily to influence the surface finish in the decorative area. This process step can be carried out optionally depending on requirements.
Chemical pre-treatment Degreasing (E0 / DIN 17611)
Components are usually cleaned in alkaline degreasing baths containing surfactants. This process is extremely important to remove dirt, oil and machining emulsions such as cutting oil or coolant from components. The degreasing bath does not have an abrasive effect on the aluminum surfaces. Slight pre-corrosion or scratches, for example, cannot be removed with it. Professional pre-cleaning of the components is crucial for the quality of an aluminum oxide coating. Failure to degrease and clean properly can result in massive defects in the coating structure.
The components are always rinsed with water between all process steps. The water quality requirements depend on the application areas of the components. In most cases, fully demineralized rinsing baths are also used.
Pickling (E6 / DIN 17611)
The pickling process is carried out in most cases, but can also be an optional part of the process sequence. As a rule, alkaline aqueous solutions based on caustic soda are used for the pickling process. There are also acidic pickling baths, which are usually used specifically for cast aluminum components.
During the pickling process, the aluminum is chemically removed. Pickling removes the natural oxide layer of the aluminum and represents a further cleaning stage in the process sequence. A surface finish can also be achieved through the pickling process. Depending on how long the components remain in the pickling solution, they become more or less matt. The longer the pickling process, the more matt the surfaces become. Care must be taken not to over-pickle, as this always results in the removal of wall thicknesses or makes material structures highly visible. Components with tolerances must be precisely adjusted in order to achieve accurately fitting final dimensions. Pickling also increases the roughness of the aluminum surfaces.
Decapping
After an alkaline pickling process, the goods are neutralized in an acidic bath, usually based on sulphuric acid or nitric acid. This process step, which should not be underestimated, neutralizes the pickling coating (black coating that mainly forms on alloys with a high copper content) and alkaline residues in holes or threads.
Anodization / anodizing process
The exact structure and properties of the anodized layer are highly dependent on the specific parameters of the anodizing process. Including the electrolyte solution used,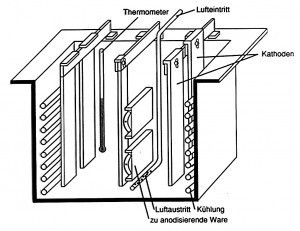 Fig. 3: Section through an anodizing bath of the current intensity, the temperature and the duration of the process. These parameters directly influence the thickness, porosity and quality of the aluminum oxide layer formed (Fig. 3). Some standard processes are briefly described below.
Fig. 3: Section through an anodizing bath of the current intensity, the temperature and the duration of the process. These parameters directly influence the thickness, porosity and quality of the aluminum oxide layer formed (Fig. 3). Some standard processes are briefly described below.
Direct current sulphuric acid anodization (GS)
The GS process is one of the most commonly used processes for producing aluminum oxide layers in the decorative area, which also meet various technical requirements. Depending on the application, layer thicknesses of between 10 and 30 µm are generally produced. The oxide layers have a low inherent coloration and can subsequently be colored excellently. Aluminum oxide coatings have very good corrosion resistance, are weather-resistant, hard and wear-resistant. Applications range from mechanical engineering, aviation and medical technology to architectural applications.
Direct current sulphuric acid oxalic acid anodization (GSX)
The GSX process, also known as hard anodizing or hard coating, is used in areas where abrasion resistance and greater hardness are crucial. Due to the process parameters, the pore size is significantly smaller than in the GS process. In addition, higher coating thicknesses can be produced due to the lower process temperatures. The standard layer thicknesses are between 35 and 50 µm and can be increased to 100 µm if required. These layers can also be colored, but the inherent coloration of the oxide layer is much more pronounced. As a result, only dark shades can usually be achieved. The inherent coloration of aluminum oxide layers is strongly dependent on the composition of the aluminum alloy and the layer thickness. For example, 6000 series aluminum alloys tend to have a medium to dark grey inherent color due to the silicon and manganese content, while the high-strength 7000 series alloys tend to have gold to brown tones due to the copper and zinc content. Depending on the alloy element, this is more or less noticeable and must be taken into account.
Phosphoric acid anodization (PAA=Phosporic Acid Anodization)
The PAA process is used as a technical oxide layer and is frequently used in aviation. In the PAA process, coarse-pored and very thin oxide layers in the range of 200 to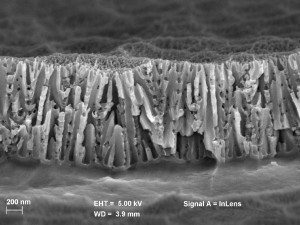 Fig. 4: Fracture surface view of the pore structure of a phosphoric acid anodized layer 1500 nanometers are produced. Due to the significantly larger pores compared to the oxide layers produced in sulphuric acid electrolytes and the firm bond with the aluminum surface, these are perfectly suited as an adhesive base for subsequent bonding or paint coatings. In aerospace technology, these oxide layers are often used in combination with a special adhesive primer for subsequent bonding (ASTM D3923-98). Due to the pore size, it is not possible to compact these layers. When a bonding primer is subsequently applied, the pores are used to ensure excellent anchoring of the primer to the oxide layer (Fig. 4).
Fig. 4: Fracture surface view of the pore structure of a phosphoric acid anodized layer 1500 nanometers are produced. Due to the significantly larger pores compared to the oxide layers produced in sulphuric acid electrolytes and the firm bond with the aluminum surface, these are perfectly suited as an adhesive base for subsequent bonding or paint coatings. In aerospace technology, these oxide layers are often used in combination with a special adhesive primer for subsequent bonding (ASTM D3923-98). Due to the pore size, it is not possible to compact these layers. When a bonding primer is subsequently applied, the pores are used to ensure excellent anchoring of the primer to the oxide layer (Fig. 4).
Coloring
After anodic oxidation, the highly porous layer can remain colorless or be colored. There are various methods for coloring anodic oxide layers, which differ in the range and quality of the color shades that can be achieved.
Dip dyeing
Chemical dyeing by immersion in inorganic or organic dye baths. In dip dyeing, color pigments are embedded in the pores of the oxide layer. The color spectrum covers a wide range of shades. With the exception of white, a wide range of colors from yellow to black can be covered. It should be noted that not all colors have the same lightfastness. In outdoor areas, only UV-resistant color pigments should be used for coloring. Otherwise, unsightly discoloration of the oxide layers may occur. The anodizing companies can provide information on this.
Electrolytic coloring
Electrolytic coloring is carried out in metal salt solutions. In this process, tin is usually electrolytically deposited from a metal salt solution at the base of the pores. The color palette here is somewhat limited and ranges from beige and brown tones to black. The coatings are extremely resistant to light and weathering.
Compacting
To ensure full corrosion and weather protection, the layers must be compacted in a further step. The colourless oxide layers are applied directly after the anodizing process, the coloured oxide layers after the colouring process. This so-called sealing or compaction process is usually carried out in hot demineralized water (98 °C). However, there are also low-temperature or cold sealing processes that have become more or less established. In this process step, the aluminum oxide is converted into boehmite (aluminum oxide hydroxide) and the existing pores are closed.
It should be noted that during the sealing process in hot water without special additives, an undesirable deposit, known as a sealing deposit or smear, can form. The water quality and pH value are also crucial in preventing this and ensuring perfect sealing quality. The conductivity and pH value of the water must therefore be checked regularly.
Influence of the alloying elements
As explained in the previous editions of the aluminum series, alloying elements have a significant influence on the material properties of aluminum. Depending on the alloying elements and the way in which the semi-finished aluminium products are manufactured, material phases are formed which are more or less noticeable during anodization. Depending on the type of phases, they can be incorporated into the oxide layer in different ways.
The alloy phases are oxidized, this is the best possible case, i.e. the phases are incorporated into the oxide layer without interference and neither influence the layer structure nor do they have an effect on the corrosion properties. If, on the other hand, the phases are dissolved during the anodizing process, cavities form in the oxide layer which, depending on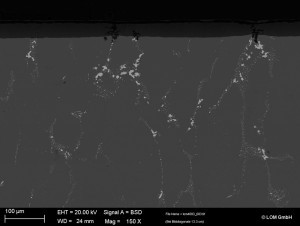 Fig. 5: Cross-section scanning electron micrograph according to size and position, can have a massive influence on the coating properties and resistance. Figure 4 shows the cross-section of an oxide layer with a high copper content (> 6% Cu). The material phases extremely disrupt the structure of the aluminum oxide layer, forming large cavities that extend to the pore base and base metal. This has a massive impact on the corrosion resistance and mechanical properties of the components. Some types of material phases are incorporated into the oxide layer undissolved and unoxidized. They are surrounded and enclosed by the oxide layer. These phases can also have a considerable influence on the coating properties depending on their size and position, as well as the phase composition (Fig. 5).
Fig. 5: Cross-section scanning electron micrograph according to size and position, can have a massive influence on the coating properties and resistance. Figure 4 shows the cross-section of an oxide layer with a high copper content (> 6% Cu). The material phases extremely disrupt the structure of the aluminum oxide layer, forming large cavities that extend to the pore base and base metal. This has a massive impact on the corrosion resistance and mechanical properties of the components. Some types of material phases are incorporated into the oxide layer undissolved and unoxidized. They are surrounded and enclosed by the oxide layer. These phases can also have a considerable influence on the coating properties depending on their size and position, as well as the phase composition (Fig. 5).
The anodization of magnesium and silicon-containing aluminium materials of the 5000 and 6000
alloy groups such as EN AW 5083 (AlMg4.5Mn), EN AW 5005 (AlMg1) or EN AW 6061 (AlMg1SiCu) and EN AW 6082 (Al Si1MgMn) can be protected very well using standard processes. These aluminum alloys are mostly used in mechanical and apparatus engineering or in decorative applications (architecture). The inherent coloration of these alloy groups of aluminium alloys is relatively low and shifts into the grey tone range at higher layer thicknesses. High-strength aluminum alloys such as EN AW 2024 (AlCu4Mg1) or EN AW 7075 (AlMgZnCu1.5), on the other hand, sometimes have a high copper content. Aluminium alloys with a very high copper content are usually more difficult to anodize. Special process parameters are required for copper contents > 3 % in order to sufficiently coat the aluminum parts. High-alloy aluminum materials or cast plates are often used in the automotive and aerospace industries as well as in mechanical engineering and mold making. In particular, the aluminum alloys EN AW 2024 (main alloying element copper) and EN AW 7075 (main alloying element zinc) are frequently used due to their high strength and high-temperature resistant material properties. However, a high amount of alloying elements in the material can also have some disadvantages. EN AW 2024 (AlCu4Mg1), for example, has poor corrosion resistance and it is very difficult to achieve oxide layer thicknesses of more than 10-15 µm in standard anodizing processes (sulphuric acid). EN AW 7075 (AlZn5.5MgCu) has good anodizing properties but low corrosion resistance with a trend towards stress corrosion cracking.
The high-alloy copper-containing aluminium materials in particular have a tendency to "burn" during the anodizing process. The so-called "burning" describes a very strong re-dissolution effect on the component. This effect is supported by partial overheating and stress peaks at component edges. The component is partially attacked and dissolved. In the worst case, this can lead to the complete destruction of a component.
To avoid this, the parameters of an anodizing process must be adapted to the respective aluminium alloy. This means that even high-strength aluminium alloys can be anodized very well in order to meet the technical requirements of the coating systems 100%.
Redissolving effect
The layer thicknesses produced are subject to a law. During anodization or the anodizing process, the aluminium oxide layer is built up from the base material. However, the electrolyte, the temperature and the process parameters of current and voltage also cause the oxide layer to re-dissolve. As long as the anodization rate is greater than the redissolution rate, a layer build-up takes place. At a certain point, however, this balance shifts in favor of the redissolution rate. Even if the anodizing process is continued from this point, the oxide layer does not build up any further in thickness. The pores are widened and the oxide layer is dissolved back. Experience has shown that layer thicknesses of 5 to 35 µm are produced in the GS process, 30 to 100 µm in the GSX process and 200 to 1500 nm in the PAA process (Fig. 6).
 Fig. 6: Diagram of layer growth
Fig. 6: Diagram of layer growth
Areas of application
Over the course of time, anodizing has developed into an important process in various industrial sectors. Today it is used in the automotive, aerospace, construction, electronics and many other industries. Anodizing processes include a variety of techniques to precisely control and adjust the coating thickness, surface finish, color effects and other properties of the anodized surface.
Facade technology / building envelopes
Aluminum oxide coatings provide an aesthetically pleasing surface with excellent weather resistance, making them ideal for building facades, railings, window frames and other architectural applications (Fig. 7).  Fig. 7: Facade cladding with anodized aluminum
Fig. 7: Facade cladding with anodized aluminum
Automotive industry
Anodized surfaces are indispensable in the automotive industry. This technology improves the performance of aluminum components in several ways. The aluminum oxide layer provides effective corrosion protection, increases wear resistance in stressed areas and enables decorative surface designs. From body parts to emblems, anodizing contributes to the durability, aesthetics and performance of modern vehicles.
Aerospace
Aluminium has successfully established itself in the aerospace industry, particularly through the anodization of components, which provides effective protection against corrosion. This surface treatment is ideal for interior components such as seat rails and structurally bonded parts. Anodization also serves as a thin-layer pre-treatment of painted surfaces and as corrosion protection and primer. Phosphoric acid anodization is a special process that is ideally suited as a primer. Compared to chromic acid anodizing, which should no longer be used due to its chromium(VI) components, which are classified as harmful to health, phosphoric acid anodizing offers a genuine and approved alternative.
Electronics and mechanical engineering
Aluminum oxide coatings set new standards in electronics by providing an insulating protective layer. This makes them ideal for use in housings, heat sinks and other components. In mechanical engineering, on the other hand, the focus is on abrasion resistance, corrosion resistance and scratch resistance, while the insulating effect of this treatment effectively counteracts galvanic corrosion.
However, if electrical conductivity is required, areas can be masked accordingly. Components can thus be provided with an insulating layer at the same time and still have an electrical discharge at the masked areas (Fig. 8). Fig. 8: Coloured anodized impeller with bare contact surface
Fig. 8: Coloured anodized impeller with bare contact surface
Food industry / medical technology
The versatility of aluminum oxide coatings even extends into the food sector, where they are successfully used in production machinery. In the fascinating world of medical technology, anodized aluminium components play a decisive role - be it in precise dental instruments or in highly sensitive operating theatres. This is where innovation and healthcare merge.
Conclusion
The growth of the oxide layer out of the aluminium and the strong bond with the aluminium is what makes this coating system and the anodizing process so exceptional. The variety of possible layer structures due to different process parameters offers a multitude of advantages. These layers effectively protect the aluminum from corrosion and environmental influences, giving the surface hardness and resistance to wear. The possibility of different colors opens up a wide range of aesthetic options. Due to their versatility in combining and fulfilling technical and decorative requirements, aluminum oxide coatings have become a part of our lives.
Although anodizing processes have been used industrially for many decades, the research and development of new, optimized processes and coating properties is far from over.
The development of powder and wet paint is also constantly being optimized and covers a broad field in our daily applications. Read more about this in the next issue of our aluminum series.


