Environmentally conscious politicians want to limit the use of PFAS chemicals to the bare minimum, while manufacturers are warning against a comprehensive ban. The ban on perfluorinated and polyfluorinated alkyl compounds (PFAS) being discussed in the EU would pose an enormous threat to high-tech industries such as medical and semiconductor technology, several associations have warned. The fact is: PFAS are substances that have already accumulated to an alarming degree all over the world, right up to the Antarctic. A closer look at the perpetual chemicals is therefore advisable.
PFAS are aliphatic organic substances in which all hydrogen atoms on at least one carbon atom are completely replaced by fluorine atoms. As they and their degradation products are very persistent in the environment, they are also known as "eternity chemicals" [1]. The PFAS chemical group is estimated to include over 10,000 individual substances that are used in everyday products such as anoraks, pans and cosmetics (Fig. 1). In industry, they are used in seals, insulation and cables, among other things. Lithium-ion batteries, for example for electric cars, also rely on PFAS. What type of compounds are these? Let's take a closer look!
 Fig. 1: Examples of the wide range of PFAS applications (Graphics: Wolfgang Hasenpusch)
Fig. 1: Examples of the wide range of PFAS applications (Graphics: Wolfgang Hasenpusch)
Damage to health caused by PFAS
Some PFAS are suspected of being carcinogenic. The total annual health-related costs associated with exposure to PFAS were estimated to be at least 52 to 84 billion euros for the European Economic Area (EEA) countries in 2019 [2] and 6 to 62 billion US dollars for the United States in 2018 [3]. The total annual cost of environmental screening, contamination monitoring, water treatment, soil remediation and health assessment in the EEA plus Switzerland is between €821 million and €170 billion [2]. The American Water Works Association estimates that it would cost 370 billion US dollars to remove PFAS from US drinking water [4]. Taking into account the externalized social costs, the cost per kilogram of PFAS would be around 18,700 euros, while the actual average market price of PFAS is around 19 euros per kilogram [5].
People can ingest PFAS primarily through drinking water and food. PFAS are introduced into our food in various ways: they can be detected in soil, drinking water, animal feed and consumer goods such as packaging.
According to current knowledge from the European Food Safety Authority (EFSA), animal foodstuffs in particular are contaminated with PFAS. In 2020, the EFSA recommended that a group-related tolerable weekly intake (TWI) of 4.4 nanograms per kilogram of body weight should not be exceeded for the sum of the four predominant PFAS in human blood. These are perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorononanoic acid (PFNA) and perfluorohexane sulfonic acid (PFHxS).
The recommendation is based on observed effects on the immune system of infants. Since January 1, 2023, legally binding maximum levels for the four PFAS in the EFSA opinion have applied throughout the EU - individually and as a sum - in fish and fishery products, crustaceans and molluscs, meat and game, eggs and products made from them [6]. In addition, the European Commission recommends that a large number of frequently consumed foods such as fruit, vegetables, cereals and baby food for infants and young children should be monitored for PFAS [7]. The ongoing monitoring of drinking water contamination is the responsibility of the Federal Ministry of Health [8].
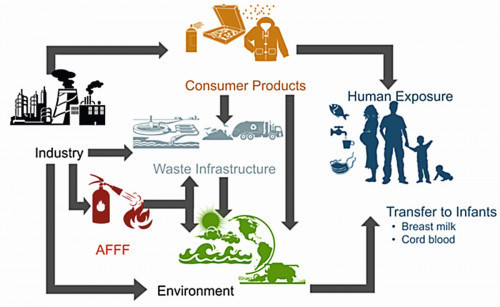 Fig. 2: Environmental effects of PFAS
Fig. 2: Environmental effects of PFAS
Figure 2 shows a graphical representation of how PFAS enter our bodies, with the global distribution via the air and water pathways and the uptake by young children being of particular concern [9]. Figure 3 illustrates the extent to which the incorporation of PFAS can cause damage to women, men and, above all, children in the womb.
PFAS substances in Germany
The German Environmental Health Study (GerES) also found widespread exposure among children and adolescents. EU-wide studies of adolescents between 2016 and 2022 as part of the "HBM4EU" research initiative showed that the blood concentration on which the EFSA TWI value is based was exceeded in an average of 15% of all studies, and in individual studies in over 20% of participants.
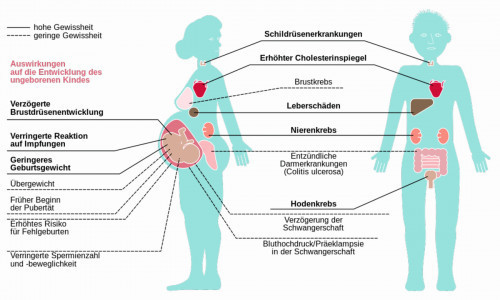 Fig. 3: Health effects of exposure to PFAS
Fig. 3: Health effects of exposure to PFAS
The Federal Ministry for the Environment does not have any financial resources to support the removal of PFAS contamination, as the responsibility for implementing the remediation of contaminated sites lies with the federal states. Nevertheless, the ministry is tackling the problem of PFAS together with the federal states, e.g. through enforcement aids for assessment and remediation as well as in the evaluation of proportionate, pragmatic remediation options:
- Working aid on "Remediation management for local and areal PFC contamination"
- PFAS guideline on "Recommendations for the assessment of soil and water contamination and for the disposal of PFC-containing materials" [10].
The Federal Ministry for the Environment is also funding various PFAS projects through its departmental research program. One of the aims is to advance the establishment of test and action values in soil protection legislation and to ensure legal certainty for the enforcement authorities. To this end, the improvement of the data situation and the assessment basis must be strengthened in cooperation with the federal states and in European exchange [8].
To date, there has been no official presentation of nationwide cases of damage. Stadtwerke Rastatt, which is affected by one of the largest PFAS damage cases in Germany, has compiled an overview of damage cases in Germany through queries and online research. This can be viewed on the Stadtwerke website. It is a non-official list that makes no claim to completeness or accuracy [11].
In and around the production sites, there is probably the greatest risk of massive environmental contamination. These factories are located in Bad Wimpfen, in Frankfurt/M., in Leverkusen and in the Bavarian chemical park Gendorf near Burgkirchen an der Alz, where three PFAS producers have set up shop [12].
So far, the public has mainly been discussing a few PFAS hotspots. Fields in Rastatt in Baden-Württemberg, for example, where suspected contaminated paper sludge was spread. Or about Düsseldorf Airport, where extinguishing foam containing PFAS flowed into the soil and groundwater during a major fire on April 11, 1996.
PFAS are still widely used in fire-fighting foams. The Federal Environment Agency (UBA) recommends the use of fluorine-free alternatives due to their worrying properties [13]. Extinguishing agents containing fluorine should only be used for fires for which no sufficiently effective alternatives are currently available, such as in aviation.
Bans on PFAS
Even before the EU REACh Regulation came into force in 2006, an EU-wide ban on perfluorooctane sulfonic acid (PFOS) was adopted [14], which was incorporated into the EU POP Regulation (POP = persistent organic pollutants) shortly afterwards. As a result, the PFOS-REACh entry was also deleted. In 2019, the PFOS ban was re-examined in accordance with the Stockholm Convention and all exemptions granted in the EU up to that point were removed, with the exception of the use of PFOS as a spray suppressant for non-decorative hard chrome plating with carcinogenic chromium(VI) in closed circulation systems.
The particularly relevant perfluorooctanoic acid (PFOA) was initially regulated throughout the EU, including its salts and precursor compounds, on the initiative of the German authorities in cooperation with the Norwegian authorities.
At the same time, the inclusion of PFOA in the global ban list of the Stockholm Convention on Persistent Organic Pollutants was promoted and adopted in 2019.
The regulation in the EU POP Regulation has been in force since December 2020 with various deadlines until December 2036 at the latest for various exemptions to enable a switch to suitable alternatives. The exemptions include uses for implantable medical devices, fire-fighting foams, photographic coatings and photolithographic processes, oil and water-repellent textiles and industrial polymers for specific membranes or sealants, as no suitable alternatives are yet available. In addition, perfluorohexane sulfonic acid (PFHxS) was included in the Stockholm Convention as a further POP in 2022.
From February 2023, the placing on the market, production and use of perfluorinated carboxylic acids with nine to fourteen carbon atoms (PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA) will also be restricted. The EU Commission is also currently working on a proposal to regulate perfluorohexanoic acid (PFHxA). A supplementary proposal to regulate fire-fighting foams containing fluorine is currently being evaluated by the scientific committees at the European Chemicals Agency (ECHA). A decision is expected in 2024.
Various other PFAS such as perfluorobutane sulfonic acid and "GenX" (ammonium 2,3,3,3-tetrafluoro-2-propanoate) have already been identified as Substances of Very High Concern (SVHC) under REACh and included in the corresponding SVHC list in order to substitute these as well [8].
Selected PFAS substance classes
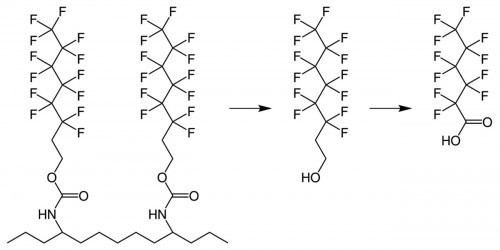 Fig. 4: In side-chain fluorinated polymers, fluorotelomers can be detached from the polymer backbone by hydrolysis and converted to perfluorocarboxylic acidsPFASare not completely degradable under environmental conditions, or only over very long periods of time. Certain groups of PFAS are converted into stable PFAS by environmental processes such as hydrolysis, oxidation, decarboxylation, reduction and hydroxylation (Fig. 4). For example, not fully fluorinated compounds such as fluorotelomers including fluorotelomer alcohols or perfluoroalkylsulfonamides (such as perfluorooctanesulfonamide) are converted into perfluorocarboxylic acid or perfluorosulfonic acids, which have an extremely high persistence [15]. According to the OECD, there are at least 4730 different PFAS with at least three perfluorinated carbons. The CompTox Chemic as the control center of the US Environmental Protection Agency (EPA) contains 14735, PubChem even around six million PFAS. Over 1400 PFAS could be assigned to more than 200 different applications. Some types of fluorinated polymers are
Fig. 4: In side-chain fluorinated polymers, fluorotelomers can be detached from the polymer backbone by hydrolysis and converted to perfluorocarboxylic acidsPFASare not completely degradable under environmental conditions, or only over very long periods of time. Certain groups of PFAS are converted into stable PFAS by environmental processes such as hydrolysis, oxidation, decarboxylation, reduction and hydroxylation (Fig. 4). For example, not fully fluorinated compounds such as fluorotelomers including fluorotelomer alcohols or perfluoroalkylsulfonamides (such as perfluorooctanesulfonamide) are converted into perfluorocarboxylic acid or perfluorosulfonic acids, which have an extremely high persistence [15]. According to the OECD, there are at least 4730 different PFAS with at least three perfluorinated carbons. The CompTox Chemic as the control center of the US Environmental Protection Agency (EPA) contains 14735, PubChem even around six million PFAS. Over 1400 PFAS could be assigned to more than 200 different applications. Some types of fluorinated polymers are
Fluoropolymers
- Polytetrafluoroethylene (PTFE) = Teflon
- Polyvinylidene fluoride (PVDF)
- Fluorinated ethylene propylene
- Perfluoroalkoxy polymer (PFA)
Side-chain fluorinated polymers
- Fluorinated methyl acrylic polymers (fluorotelomer acrylate (FTA))
- Fluorinated urethane polymers
- Fluorinated oxetane polymers
Other polymers
Perfluoropolyethers (PFPE)
The structure and properties of the four most common compounds perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), perfluorononanoic acid (PFNA) and perfluorohexanesulfonic acid (PFHxS) are discussed in more detail below:
Perfluorooctanoic acid, PFOA
PFOA is referred to as an "eternal chemical" because it never degrades in the environment (Fig. 5). Due to its classification as a CMR substance (C stands for carcinogenic, M for mutagenic and R for reprotoxic) and PBT substance (P stands for persistent, B for bioaccumulative and T for toxic), the manufacture and placing on the market of PFOA and precursor compounds has been banned in the EU since 2020 and in Switzerland since 2021, with a few exceptions [16].
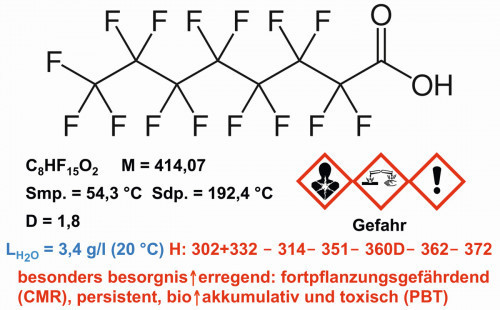 Fig. 5: Structure and properties of perfluorooctane carboxylic acid
Fig. 5: Structure and properties of perfluorooctane carboxylic acid
PFOA has oil and water-repellent properties. The high stability and resistance of perfluorooctanoic acid under a wide range of conditions justify its suitability for various applications. Perfluorooctanoic acid is now a widespread substance in the environment, as it is both persistent and bioaccumulative. In water, the compound can be degraded under sunlight with the help of a boron nitride-titanium dioxide composite.
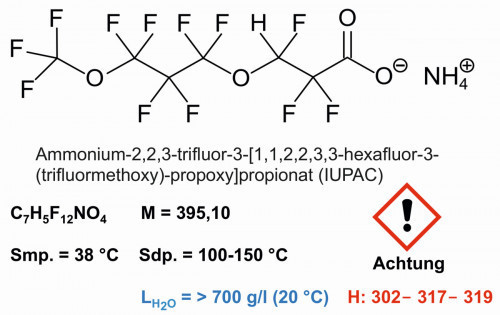 Fig. 6: Structural formula and properties of ADONA
Fig. 6: Structural formula and properties of ADONA
Perfluorooctanoic acid in the form of ammonium perfluorooctanoate was mainly used as an emulsifier for the production of polymers such as Teflon (Chemours) and polytetrafluoroethylene. Substitutes such as ammonium 2,2,3-trifluoro-3-[1,1,2,2,3,3-hexafluoro-3-(trifluoromethoxy)-propoxy]propionate (Fig. 6) are now used in these processes.
Perfluorooctane sulfonic acid, PFOS
The compound was usually marketed as a potassium, lithium, ammonium, diethanolammonium or tetraethylammonium salt. PFOS was included as a pollutant in Annex B of the Stockholm Convention in 2009 (Fig. 7).
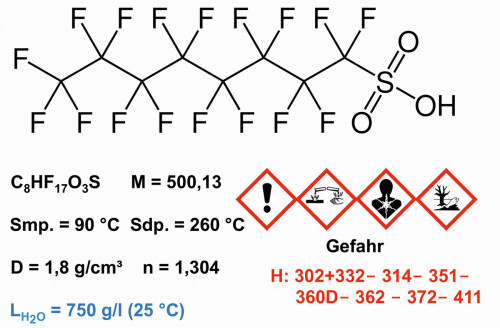 Fig. 7: Structure and properties of perfluorooctane sulfonic acid
Fig. 7: Structure and properties of perfluorooctane sulfonic acid
In 1953, the chemist Patsy O'Connell Sherman (1930-2008) accidentally discovered the cleaning effect of a fluoropolymer at 3M. She and Samuel Smith (1927-2005) brought perfluorooctane sulfonate to product maturity by 1956 [17]. In 1989 Sherman was the first woman to be inducted into the Minnesota Hall of Fame and in 2001, together with Samuel Smith, into the National Inventors Hall of Fame.
PFOS exposure is associated with approximately 382,000 deaths per year among US adults in the US alone for the period from 1999 to 2015. For the period from 2015 to 2018, the number fell to around 69,000 per year. Primary causes of death were heart disease and cancer [18].
Perfluorononanoic acid, PFNA
Perfluorononanoic acid is a flammable, hardly flammable, crystalline, beige-colored solid that is practically insoluble in water (Fig. 8). There are theoretically 89 skeletal isomers of PFNA. Long-chain perfluoroalkanecarboxylic acids (such as perfluorononanoic acid) and their salts are surface-active chemicals (surfactants) that strongly reduce the surface tension of water, aqueous solutions and organic liquids even in low concentrations. These acids (C6-C12) and their derivatives are used as wetting, dispersing, emulsifying and foaming agents.
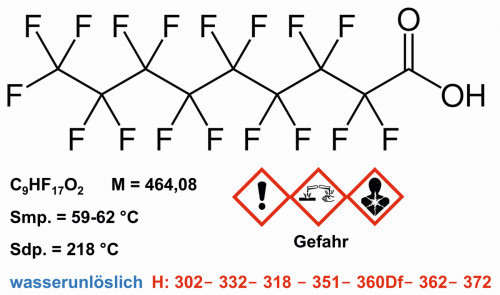 Fig. 8: Structure and properties of perfluorononanoic acid, PFNA
Fig. 8: Structure and properties of perfluorononanoic acid, PFNA
Perfluorononanoic acid was placed on the candidate list of substances of very high concern by the ECHA in Helsinki due to its reprotoxic and PBT properties. Under the leadership of the German and Swedish authorities, a ban on perfluorocarboxylic acids with chain lengths C9 to C14 was drawn up. The corresponding EU regulation came into force in 2021. The ban on the manufacture, placing on the market and use has been in force since February 2023, with longer transitional periods for certain uses. A similar ban came into force in Switzerland in 2022 [19].
Perfluorohexane sulfonic acid, PFHxS
PFHxS was produced by electrochemical fluorination. This produces around 95 % of the linear isomer. Theoretically, there are 17 skeletal isomers of PFHxS (Fig. 9).
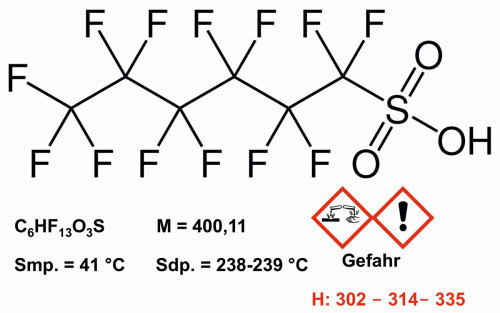 Fig. 9: Structure and properties of perfluorohexanesulfonic acid
Fig. 9: Structure and properties of perfluorohexanesulfonic acid
PFHxS has been used in foam extinguishing agents (AFFF = Aqueous Film Forming Foam), as an anti-fogging agent in chrome plating, in impregnating agents for textiles, leather and upholstered furniture (e.g. in "Scotchgard"), in polishing, cleaning and washing agents, in coatings and in the manufacture of semiconductors [20]. PFHxS was nominated for inclusion in the Stockholm Convention in 2017 and added to its Annex A in 2022. Several hundred salts and precursors of PFHxS are covered by the ban. In Switzerland, production, placing on the market and use have been banned since October 2022 [21].
|
Acids |
M |
Smp [°C] |
Sdp. [°C] |
Density |
H-phrases |
|
PF-propionic acid |
164,03 |
-35 |
97 |
1,576 |
314 |
|
PF-butanoic acid |
214,04 |
-18 |
120 |
1,645 |
314 |
|
PF-pentanoic acid |
264,05 |
0 |
140 |
1,713 |
318-361d |
|
PF-hexanoic acid |
314,05 |
14 |
157 |
1,759 |
314 |
|
PF-heptanoic acid |
364,06 |
30 |
175 |
1,79 |
360D-372 |
|
PF-octanoic acid |
414,07 |
54 |
192 |
1,8 |
302+332-314-351-360D-362-372 |
|
PF-nonanoic acid |
464,08 |
62 |
218 |
1,8 |
302-332-318-351-360Df-362-372 |
|
PF-decanoic acid |
514,08 |
81 |
230 |
1,8 |
301-351-360Df-362 |
|
PF-undecanoic acid |
564,09 |
101 |
245 |
1,8 |
314 |
|
PF-tetradecanoic acid |
714,12 |
132 |
300 |
1,8 |
314 |
No complete data sets are available for either the polyfluoroalkanoic acids or the analogous representatives of the polyfluoroalkanesulfonic acids. For the through-fluorinated alkanecarboxylic acids, the available data for melting and boiling points and densities can be used to interpolate missing data, at least graphically, and fill the gaps (marked in red) in Table 1. The same applies to the densities, some of which only result from estimates. Due to the corresponding lack of parameters, the situation is even worse for polyfluoroalkanesulfonic acids. The few available values only allow a rough estimate.
Orientation and limit values
In view of the large number of compounds, only very powerful separation methods such as HPLC (high-performance liquid chromatography) coupled with mass spectrometers can be used for reliable qualitative and quantitative determination of the substances after extensive sample preparation.
Although the danger posed by PFAS has been known for years, there is still no obligation for water suppliers to test the drinking water they provide accordingly. As a result, there are no reliable nationwide data sets on possible PFAS contamination in the water we consume every day. The German Drinking Water Ordinance does not contain any specific limit values for the PFAS group (PFOA, PFOS, etc.). The Federal Environment Agency recommended the following maximum values in 2006 [1]:
- 100 ng/L: "Health orientation value" - target value for drinking water for lifelong exposure
- 300 ng/L: "Guideline value tolerable for lifelong exposure for all population groups"
- 500 ng/L: "Precautionary action value for infants and pregnant women"
- 5 μg/L: "Action value for adults" - "No longer usable" as drinking water Values of 1.5 to 5 μg/L can only be tolerated for up to 1 year.
From 2026 or 2028, the following limit values should apply:
- 100 ng/L: Total PFAS (perfluorocarboxylic acids and perfluorosulfonic acids with carbon chain lengths of 4 to 13)
- 20 ng/L: sum PFAS-4 (PFOA, PFNA, PFHxS, PFOS)
The recast of the European Drinking Water Directive, Directive (EU) 2020/2184 of December 16, 2020, requires the use of summary approaches and, since January 12, 2024, the Commission has issued technical guidance on analytical methods for monitoring
of PFAS within the parameters PFAS total and sum of PFAS, including detection limits, parameter values and frequency of sampling. The minimum requirements are:
- 500 ng/L: PFAS total (sum of all PFAS)
- 100 ng/L: sum of PFAS (sum of 20 PFAS - perfluorocarboxylic acids and perfluorosulfonic acids with carbon chain lengths from 4 to 13)
The Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) classified the minimum requirement of 0.5 μg/L for the sum of all PFAS as clearly too high. To protect against adverse health effects from the consumption of fish contaminated with PFOS, the environmental quality standard for PFOS in inland surface waters was set at 0.65 ng/L as an annual average by the Water Framework Directive.
If the following guideline values are exceeded in foodstuffs in the EU, an investigation into the causes of the contamination should be carried out:
- 10 ng/kg for PFOS, 10 ng/kg for PFOA, 5 ng/kg for PFNA and 15 ng/kg for PFHxS in fruit, vegetables and starchy roots and tubers
- 1.5 μg/kg for PFOS, 10 ng/kg for PFOA, 5 ng/kg for PFNA and 15 ng/kg for PFHxS in wild mushrooms
- 20 ng/kg for PFOS, 10 ng/kg for PFOA, 50 ng/kg for PFNA and 60 ng/kg for PFHxS in milk
- 50 ng/kg each for PFOS, PFOA, PFNA and PFHxS in complementary food
Summary and commentary
It is now clear that it was irresponsible to produce fluorinated chemicals and thus inevitably release them into the environment, both during production and during and after use.
These classes of compounds have wreaked incalculable havoc around the world: Many people suffer from the consequences and die an early death through no fault of their own. Entire stretches of land lie contaminated and fallow, passing on their cruel legacy to millions of earthlings. Lurking in the soil, water and air are the imperishable, ill-fated fluorochemicals that will continue to harm many generations - now also through food. But the legislators cannot think of anything more than monitoring and setting limits in environmental regulations. Protecting people from further harm requires more: bans on exemptions, alternative methods, decontamination procedures!
Instead of stopping their scandalous activities, limiting the damage and doing everything possible to decontaminate their contaminated sources, the manufacturing companies are still going on the barricades and influencing politicians not to issue any bans, approve irresponsible exemptions and not to take recourse against producers.
In the case of asbestos, dioxins, warfare agents, mercury emissions, marine pollution, plasticizers in plastics, microplastics and CFCs, to name just a few of the dark sides of chemistry, manufacturers had already largely shirked their responsibility to society.
Why don't manufacturers and polluters pay for special water filtration, decontamination of fire department training areas and production zones? Why are state permits for such hazardous substances not issued with long-term liability waivers? Why has the PFAS debacle remained in the gray area for so long?
Literature
[1] https://de.wikipedia.org/wiki/Per-_und_polyfluorierte_Alkylverbindungen
[2] Nordic Council of Ministers: THE COST OF INACTION: A socioeconomic analysis of environmental and health impacts linked to exposure to PFAS, TemaNord 2019:516.
[3] Obsekov, V., L. G. Kahn, L. Trasande: "Leveraging Systematic Reviews to Explore Disease Burden and Costs of Per- and Polyfluoroalkyl Substance Exposures in the United States", Exposure and Health, July 26, 2022
[4] https://www.politico.com/news/2022/09/13/the-battle-over-who-pays-to-clean-up-chemicals-00056136
[5] https://amp-theguardian-com.cdn.ampproject.org/c/s/amp.theguardian.com/environment/2023/may/12/pfas-forever-chemicals-societal-cost-new-report
[6] EU Regulation: EU 2022/2388
[7] EU Recommendation: EU 2022/1431
[8] https://www.bmuv.de/faqs/per-und-polyfluorierte-chemikalien-pfas
[9] Sunderland et al, Journal of Exposure Science & Environment, Epidemiology 29/2 (2019)
[10] https://www.bmuv.de/themen/bodenschutz/boeden-und-ihre-belastung/belastung-von-boeden-durch-pfas-und-pfc#c54994
[11] https://www.stadtwerke-rastatt.de/pfc-schadensfalluebersicht
[12] https://www.sueddeutsche.de (23.02.2023)
[13] https://www.umweltbundesamt.de/themen/chemikalien/chemikalien-reach/stoffgruppen/per-polyfluorierte-chemikalien-pfc/pfc-in-feuerloeschmitteln
[14] EC Directive 2006/122
[15] Evich, M. et al.: "Per- and polyfluoroalkyl substances in the Environment", Science 375 (2022) No. 2
[16] https://de.wikipedia.org/wiki/Perfluoroctansaeure
[17] https://de.wikipedia.org/wiki/Perfluoroctansulfonsaeure
[18] Wen, X., M. Wang, X. Xu, T. Li: "Exposure to Per- and Polyfluoroalkyl Substances and Mortality in U.S. Adults: A Population-Based Cohort Study", Environmantal Health Perspectives, 130/6 (2022) 067007
[19] https://de.wikipedia.org/wiki/Perfluornonansaeure
[20] https://de.wikipedia.org/wiki/Perfluorhexansulfonsaeure
[21] Swiss Ordinance on Risk Reduction related to the Use of certain particularly dangerous Substances, Preparations and Articles (Chemical Risk Reduction Ordinance, ORRChem) - Annex 1.16. (10.10.2022)
Graphics: Wolfgang Hasenpusch


