In plastic electroplating, electroless plating is the first conductive layer on the substrate and has been a tried and tested process for decades. Due to stricter regulations in environmental protection and occupational safety, the requirements for processes and process steps in electroplating are continuously increasing, especially in the area of the electroless nickel process. The processes most frequently used in plastic electroplating are based on ammonium, as these processes have proven to be very stable, with a long service life and, on the other hand, deposit a homogeneous, evenly formed layer that has a positive influence on the subsequent process steps.
In plastic electroplating, the electrolessly deposited electroless nickel layer is the first conductive layer on the substrate and has been a proven process for decades. Due to stricter regulations in environmental protection and occupational health and safety, the demands on procedures and process steps in electroplating are continuously increasing, especially in the electroless nickel process. The processes most frequently used in the electroplating of plastics are based on ammonium, as these processes have proven to be very stable, with a long service life or durability, on the one hand, and on the other hand, they deposit a homogeneous, uniformly formed layer, which has a positive influence on the further process steps.
Motivation
The willingness of users (plastic electroplating companies) to rely on environmentally friendly and sustainable process steps has become increasingly apparent in recent years. Changing requirements in waste water treatment and the associated limit values as well as the requirements for occupational safety and hygiene (odor nuisance due to ammonium) make this necessary.
In order to meet these requirements, HSO has set itself the task of designing ammonium-free nickel processes that operate without external current without adversely affecting the subsequent process steps.
Over the last 15 years, the demand for electroplated plastic components - especially in the automotive industry - has increased massively.
Political influences such as REACh are also intervening in the process chain, which is encouraging the development of alternative products. As a result, process optimization for more environmentally friendly processes - while maintaining process reliability - is a constant companion for process suppliers in application technology and development laboratories.
Since 2016, EN EcoPlast has been the first ammonium-free process to complete the pioneering EcoPlast system for plastic electroplating, which is successfully in use worldwide. Based on environmental requirements and customer wishes, we have succeeded in developing a 1:1 comparable process to the ammonium-containing EN 601 system, which combines its outstanding properties (stability, 2-component performance) with ammonium-free and environmental friendliness.
Electrolyte-specific properties
Table 1 shows a basic comparison of the most important general properties of the electroless nickel systems, EN EcoPlast 601 and EN EcoPlast.
|
Parameters |
EN EcoPlast 601 |
EN EcoPlast |
|
Preparation & regeneration components |
3 |
3 |
|
Nickel concentration |
3.5 g/l |
2.5 g/l |
|
Hypophosphite concentration |
23 g/l |
13 g/l |
|
pH value |
8,5-9,5 |
8,5-9,5 |
|
Working temperature |
25-33 °C |
32-40 °C |
|
Complexation |
hard |
softer |
|
stabilization |
necessary |
necessary |
|
overflow |
yes |
yes |
|
Analyzability |
given |
given |
The main positive aspect is that both the nickel concentration and the reducing agent content are lower in the ammonium-free system. This results in lower carry-over losses and a saving of resources, which leads to cost optimization for the user.
In order to compensate for the softer complexation of the ammonium-free electrolyte, a stronger stabilization with organic and metallic stabilizers is used compared to the ammonium-containing electrolyte. As a result, the working temperature of the EN EcoPlast is approx. 5-10 °C higher than in the EN EcoPlast 601.
Both processes produce an overflow (feed and bleed), which leads to a rejuvenation of the electrolyte and therefore no accumulation of the decomposition product sodium orthophosphite. The advantage of this method is that the electrolyte can be used continuously for production over a long period of time and no new preparations are necessary at regular intervals.
The basic analyses of the main components, such as nickel, sodium hypophosphite and metallic stabilizer, do not change and remain the same for the user. For the complete analysis of the organic complexing agents in HSO EN EcoPlast, however, extended instrumental analysis is required that can detect all components.
Results of the electrolyte comparison
The results were obtained and evaluated during the development phase and have been confirmed in practical application in the series production process.
The measured values shown were determined in several separate tests and may therefore contain small differences.
The aim was to develop an ammonium-free electroless nickel process that comes close to the ammonium-containing EN EcoPlast 601 process in terms of deposition rate, stability, service life and coating composition. The results of the investigations are presented below.
In addition to the direct influencing factors on the above parameters, comparative adhesion tests were carried out in accordance with DIN 53464, for which the test specimens were coated with EN EcoPlast and EN EcoPlast 601 as the first conductive layer.
Deposition rate as a function of exposure time, pH value and working temperature
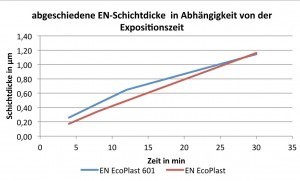
Table 2 and Figure 1 show the results in relation to the deposition rate as a function of the coating time.
|
time |
EN EcoPlast 601 - 29 °C, pH 9 |
EN EcoPlast - 40 °C, pH 9 |
||
|
4 min |
0.26 µm |
0.065 µm/min |
0.17 µm |
0.043 µm/min |
|
8 min |
0.45 µm |
0.057 µm/min |
0.35 µm |
0.043 µm/min |
|
12 min |
0.68 µm |
0.056 µm/min |
0.49 µm |
0.041 µm/min |
|
30 min |
1.14 µm |
0.038 µm/min |
1.16 µm |
0.039 µm/min |
The exposure times for the tests were 4, 8, 12 and 30 minutes on ABS plastic.
The focus is on 8 minutes, as this is the most frequently used exposure time for the electroless nickel process step. At an exposure time of 8 minutes, the layer thicknesses of EN EcoPlast and HSO EN EcoPlast 601 differ from 0.35 µm to 0.45 µm, i.e. the ammonium-containing electroless nickel process deposits approx. 25 % more layer.
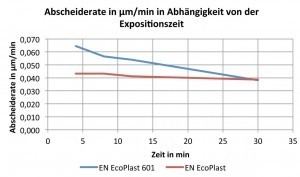
During the tests, it was found that the ammonium-containing electrolyte initially had a higher deposition rate, Figure 2, than the ammonium-free process. It can also be seen that the deposition rate of the HSO EN EcoPlast 601 decreases from 0.065 µm/min to 0.038 µm/min with increasing exposure time, while the deposition rate of the HSO EN EcoPlast remains the same at approx. 0.04 µm/min, regardless of the exposure time.
Due to the observed decrease in the layer thickness of the EN EcoPlast 601 with increasing exposure time, both electroless nickel electrolytes deposited the same layer thickness at 30 min, i.e. the deposition rates are the same.
The investigations regarding the layer thickness of the deposited electroless nickel layers as a function of the pH value were also carried out on ABS plastic. The pH working range from 8.5 to 9.5 was investigated.
Based on the values summarized in Table 3, it can be seen that the EN EcoPlast electrolyte deposits the maximum layer thickness at a pH value of 9.0, see Figure 3. With the EN EcoPlast 601 electrolyte, it can be seen that the higher the pH value, the lower the deposited layer thickness.
|
pH value |
EN EcoPlast 601- 29°C, 8' |
EN EcoPlast - 40°C, 8' |
|
8,5 |
0,42 |
0,19 |
|
9 |
0,38 |
0,32 |
|
9,5 |
0,23 |
0,22 |
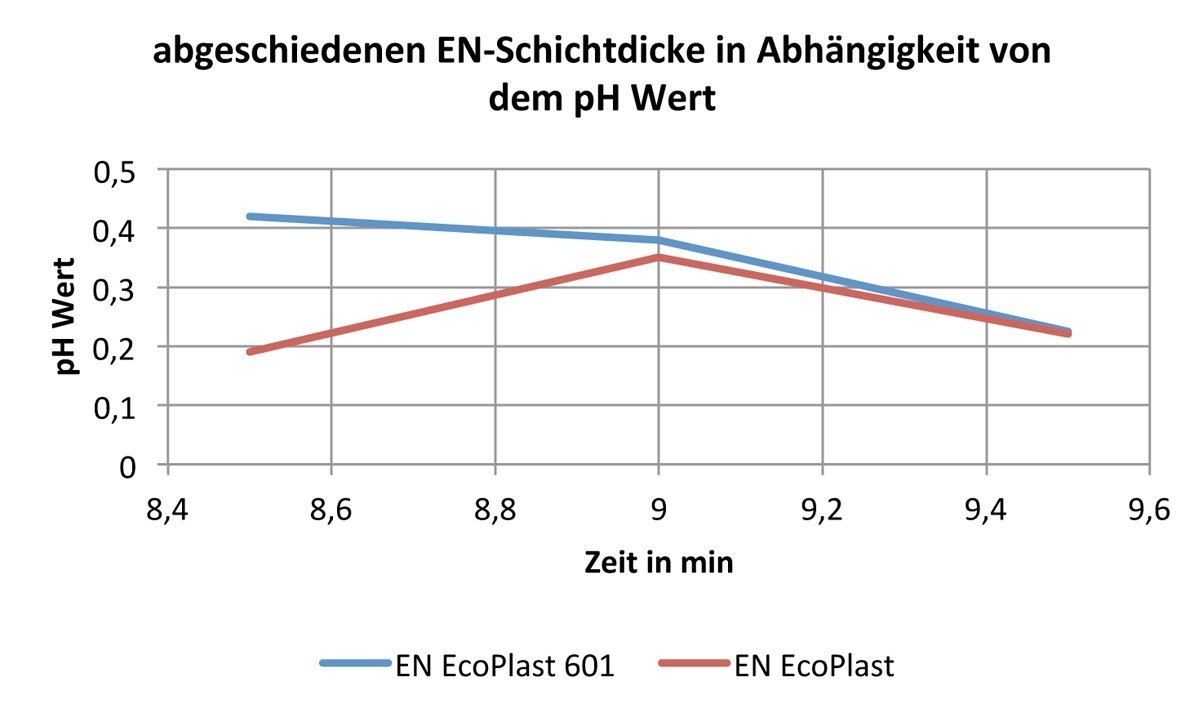 Fig. 3: Deposited EN layer thickness as a function of the pH value
Fig. 3: Deposited EN layer thickness as a function of the pH value
Overall, it can be said that both electrolytes react differently to pH changes, but deposit optimally at a pH value of 9.0 and with approximately the same layer thickness of approx. 0.3-0.4 µm without external current.
Another factor that directly influences the deposition of an electroless nickel layer is the temperature.
As previously mentioned in the electrolyte-specific comparison of properties, both electrolytes work at different temperatures. For this reason, a temperature interval of ± 5 °C was selected based on the optimum working temperature of the respective electrolyte.
The tests showed that both the EN EcoPlast and the ammonium-containing EN Ecoplast 601 electrolyte react at an increased temperature, as expected, with an increased deposition rate, which is confirmed by the values in Table 4 and shown graphically in Figure 4 .
|
Temperature in °C |
EN EcoPlast 601 - pH 9, 8' |
EN EcoPlast - pH 9, 8' |
|
25 |
0,27 |
|
|
29 |
0,38 |
|
|
35 |
0,48 |
0,2 |
|
40 |
0,32 |
|
|
45 |
0,44 |
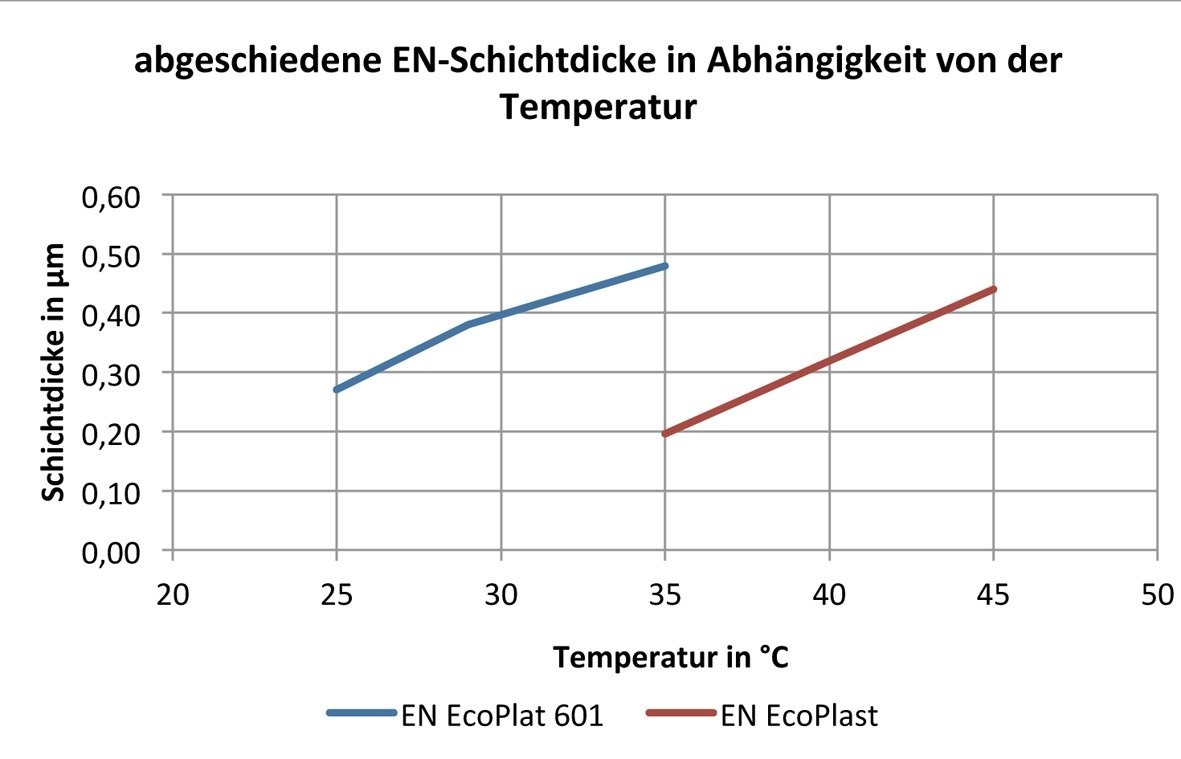 Fig. 4: Deposited EN layer thickness as a function of temperature
Fig. 4: Deposited EN layer thickness as a function of temperature
Sheet resistance and phosphorus incorporation as a function of the exposure time and the resulting EN layer thickness
In addition to the direct influencing factors of time, pH value and temperature on the activity of the electroless nickel electrolytes, the influence of the exposure time, i.e. the EN layer thickness, on the layer composition and the associated properties was also investigated.
Table 5 summarizes all the values that were determined for the layer thicknesses, the layer resistances and the phosphorus incorporation of the respective electroless nickel layer.
|
EN EcoPlast 601 - 29 °C, pH 9 |
EN EcoPlast - 40 °C, pH 9 |
|||||
|
Time in min |
ɗ in µm |
R in Ω |
P in % |
ɗ in µm |
R in Ω |
P in % |
|
4 |
0,26 |
6,0 |
4,3 |
0,17 |
18,3 |
3,8 |
|
8 |
0,45 |
2,9 |
4,0 |
0,35 |
7,0 |
3,5 |
|
12 |
0,68 |
2,3 |
3,5 |
0,49 |
3,8 |
2,9 |
|
30 |
1,14 |
0,1 |
3,0 |
1,16 |
0,9 |
2,1 |
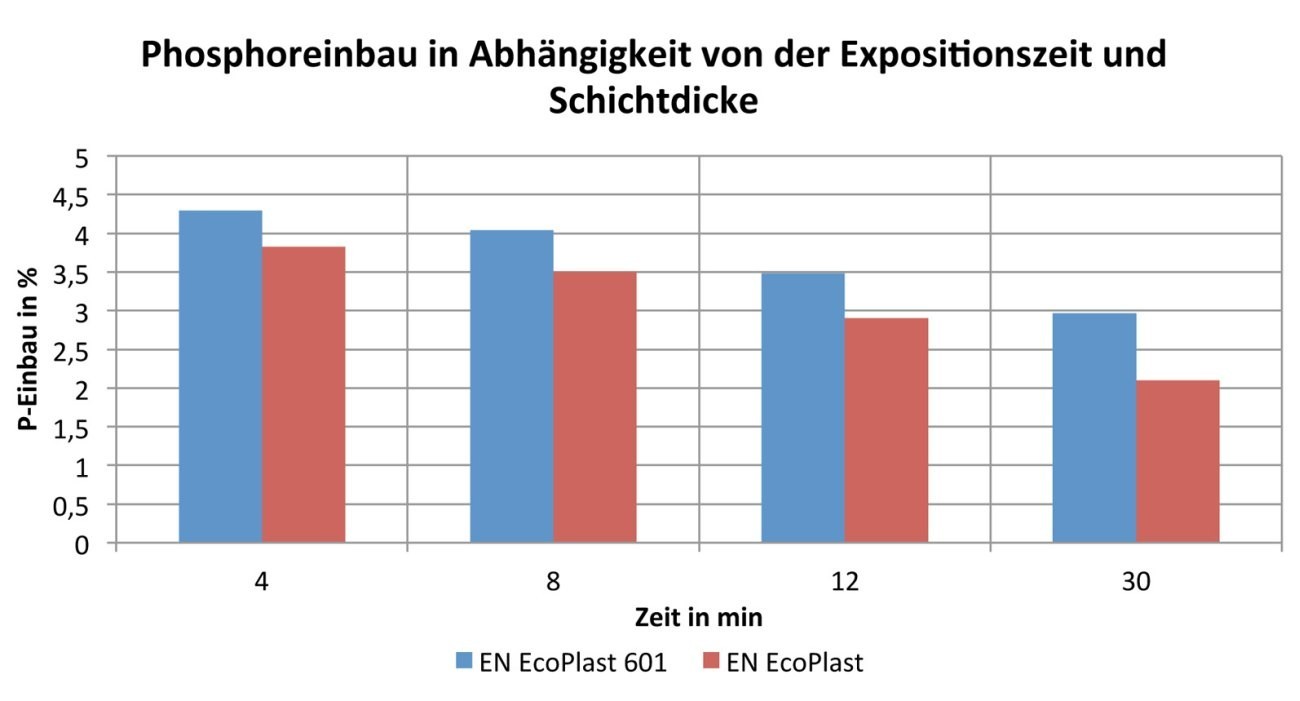 Fig. 5: Phosphorus incorporation as a function of the coating time and layer thickness
Fig. 5: Phosphorus incorporation as a function of the coating time and layer thickness
The focus here is also on the coating time of 8 minutes. If the phosphorus incorporation at 8 minutes is considered, it can be seen that this is 3.5 % for the ammonium-free HSO EN EcoPlast and 4 % for the ammonium-containing HSO EN EcoPlast 601 electrolyte. The percentage incorporation of phosphorus into the electroless nickel layer at eight minutes is therefore similar for both electrolytes, which can be seen particularly clearly in the bar chart(Fig. 5). It can be observed that the percentage of phosphorus incorporation decreases almost constantly for both electrolytes with longer coating times.
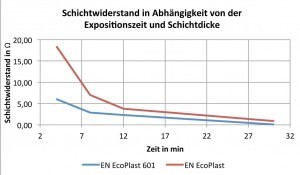 Fig. 6: Sheet resistance as a function of plating time and layer thicknessTheincorporation of phosphorus into the electroless nickel layer is important because the nickel layer forms the basis for further plating, especially if the next process step involves an immersion copper process, e.g. HSO Precodip. This is because an important charge exchange takes place between the copper ions and the electroless nickel layer. This is the only way to ensure good adhesive strength for the coating of plastic components.
Fig. 6: Sheet resistance as a function of plating time and layer thicknessTheincorporation of phosphorus into the electroless nickel layer is important because the nickel layer forms the basis for further plating, especially if the next process step involves an immersion copper process, e.g. HSO Precodip. This is because an important charge exchange takes place between the copper ions and the electroless nickel layer. This is the only way to ensure good adhesive strength for the coating of plastic components.
If the sheet resistances at 8 minutes are considered, also shown in Table 5, it can be seen that there is a difference of just under four ohms. Both sheet resistances are in the optimum range, the difference can be represented within the scope of measurement inaccuracies. The optimum range of sheet resistance for an electroless nickel layer was narrowed down to < 10 ohms in numerous preliminary tests. The graphs of both HSO EN EcoPlast 601 and HSO EN EcoPlast are shown in Figure 6.
The sheet resistance is generally an indication of the layer thickness. The higher the resistance, the thinner the coating thickness, which can be confirmed by the present tests.
Adhesive strength on ABS plastic as a function of the composite layer
The peel force measurements were carried out in accordance with DIN 53464. The constant peel force was measured at a 90° angle to the sample surface. The target values for the peel force are 7 N/cm according to current automotive standards for ABS plastic and assume a 40 µm layer of copper.
There are two ways of applying a composite layer between the electroless nickel layer and the electrolytically deposited copper layer. First, the adhesion tests with the HSO Precodip (immersion copper) composite layer are considered.
Figure 7 compares the peel force-displacement diagrams of the coated ABS sheets with the ammonium-free HSO EN EcoPlast and the ammonium-containing HSO EN EcoPlast 601 electroless nickel as the first conductive layer. The diagram on the right shows the course of the copper removal from an ABS sheet coated with the ammonium-containing HSO EN EcoPlast 601 electrolyte; a peel force of 11.3 N/cm was determined. A peel force of 9.01 N/cm was determined for the ammonium-free coated ABS sheet, as can be seen in the diagram on the left.
Both values determined are above the target value, but in a direct comparison the ABS sheet coated with HSO EN EcoPlast 601 performs slightly better, with a difference of 2 N/cm. It can be concluded from this that the ammonium-free HSO EN EcoPlast electrolyte with HSO Precodip as a composite layer could react somewhat more sensitively to the further coating.
If the peel force-displacement diagrams with the pre-nickel composite layer are considered in Figure 8, an improvement in adhesion can be seen in the evaluations. The ABS sheet coated with the EN EcoPlast 601 electrolyte now has a peel force of 12.14 N/cm and the ABS sheet coated with the EN EcoPlast as the first conductive layer also has a higher peel force of 11.03 N/cm.
Stability comparison of the electrolytes
 Fig. 9: Roughened PP sheets in electroless nickel electrolyteThestability of electroless nickel electrolytes is an important point in daily production. It usually includes practical tests to represent real, production-related situations, but also practical tests that have no points of contact with production at the user.
Fig. 9: Roughened PP sheets in electroless nickel electrolyteThestability of electroless nickel electrolytes is an important point in daily production. It usually includes practical tests to represent real, production-related situations, but also practical tests that have no points of contact with production at the user.
In the production-related example, an attempt is made to simulate cladding during operation. As can be seen in Figure 9, roughened PP sheets were immersed in the respective electrolytes during through-working in order to imitate a worn tank wall and cause premature plating. Both electroless nickel electrolytes withstood the test and no plating occurred.
Figure 10 shows the laboratory stability test in before/after images. A palladium buffer solution is required for this test.
First, a new batch of EN EcoPlast and EN EcoPlast 601 is prepared, in which all target values should be in the optimum range. The temperature for this test was set at 29 °C for both electrolyte types, as no significant difference was found in previous tests with the respective operating temperatures.
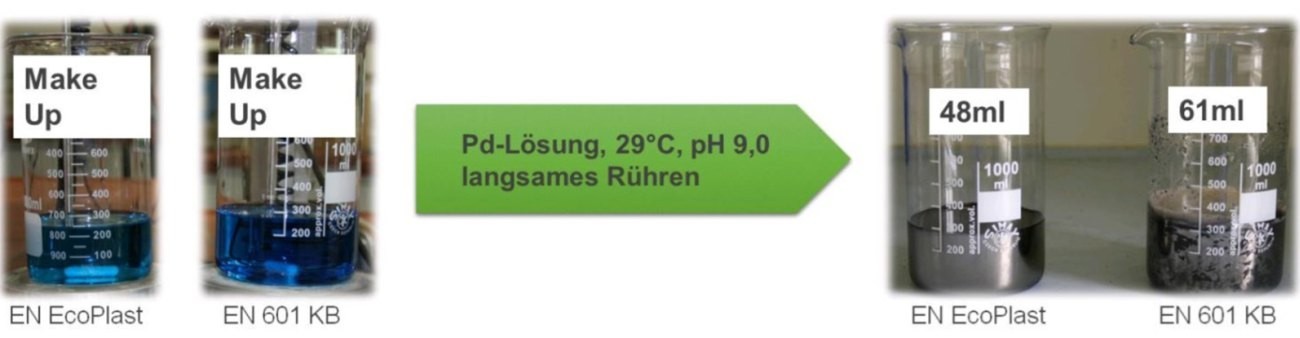 Fig. 10: Stability test of EN EcoPlast and EN EcoPlast 601
Fig. 10: Stability test of EN EcoPlast and EN EcoPlast 601
With slow stirring, one milliliter of the palladium buffer solution is added every 60 seconds until the electrolytes plate out, as shown in the right-hand image in Figure 10. Both added volumes reflect stable electrolytes - the difference is nevertheless 13 ml. From previous experiments, the most unstable electrolyte could be determined with a consumption of 25 ml, hence the statement that the new preparations of these two electrolytes can be evaluated as stable.
Waste water
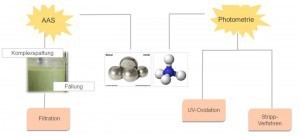 Fig. 11: Waste water diagram Electroless nickel production waste watercan contain hazardous substances and groups of substances. If wastewater containing these hazardous substances enters the sewage system, it can lead to contamination and damage to water bodies, as these pollutants cannot be sufficiently degraded in the wastewater treatment plant. They can also disrupt the operation and biological purification process of the sewage treatment plant.
Fig. 11: Waste water diagram Electroless nickel production waste watercan contain hazardous substances and groups of substances. If wastewater containing these hazardous substances enters the sewage system, it can lead to contamination and damage to water bodies, as these pollutants cannot be sufficiently degraded in the wastewater treatment plant. They can also disrupt the operation and biological purification process of the sewage treatment plant.
For this reason, there is the Wastewater Ordinance, which regulates the minimum requirements for the discharge of wastewater into bodies of water. For electroless nickel wastewater, this specifically concerns the concentrations of ammonium and nickel.
The wastewater treatments for the respective components nickel and ammonium in the ammonium-containing HSO EN EcoPlast 601 differ not only in their implementation, but also in the effort involved and the associated costs.
Atomic absorption spectroscopy, or AAS for short, is used to quantify nickel. Nickel can be dissolved very well from existing ammonium complexes, but also from other organic complexes, with the aid of complex cleavers and rebound in poorly soluble compounds. This reaction is very reliable and the consumption of the complex splitter is proportional to the nickel concentration. The newly formed, sparingly soluble compound precipitates as a precipitate and can be filtered as sludge; the waste water is nickel-free.
With regard to ammonium, photometry is used for quantification. The options for removing the ammonium from the wastewater are to carry out UV oxidation or to use the stripping process.
UV oxidation produces nitrogen as a gas, which can be discharged.
In the stripping process, the wastewater is alkalized so that the gaseous ammonia can be expelled and collected in diluted sulphuric acid, resulting in ammonium sulphate.
These treatment methods are extremely cost-intensive and time-consuming, and the ammonium sulphate produced during the stripping process must also be disposed of externally.
For the treatment of ammonium-free electroless nickel wastewater, the complete wastewater treatment that has to be carried out to remove the ammonium from the wastewater is no longer necessary. This means that the associated personnel costs and the costs for energy, e.g. in the case of UV oxidation, as well as the costs for chemicals if the treatment is carried out using the stripping process, are eliminated. In addition to the cost savings, the time required is also reduced. The resources can be used elsewhere in the company.
Summary
The following goals were achieved with the development of the ammonium-free HSO EN EcoPlast electrolyte:
- The deposition rate of HSO EN EcoPlast is comparable to the ammonium-containing HSO EN EcoPlast 601 process.
- Adhesion in combination with immersion copper processes or pre-nickel as a reinforcing layer is given for both electroless nickel systems.
- The waste water treatment of the ammonium-free HSO EN EcoPlast requires less time and effort - thus a cost saving is possible.
- The odor pollution caused by the ammonia for the employees on the systems is eliminated
Table 6 compares the advantages of the processes:
|
Product HSO EN |
Product HSO EN |
|
Higher stability in tests with Pd buffer solution |
No odor nuisance on the system, no formation of NH4Cl precipitates on the system |
|
Slightly better adhesion with Precodip |
Waste water is ammonium-free, no need for treatment |
Conclusion
The ammonium-free HSO EN EcoPlast can definitely replace the ammonium-containing electrol nickel process HSO EN EcoPlast 601 with the same requirements.
The handling differs only slightly, so only a short training period is necessary. There are differences in some parameters, which is why modifications will have to be made in the system compared to the ammonium-containing EN EcoPlast 601 system. However, this must be considered on a system-specific basis.